Page 273 - Handbook Of Multiphase Flow Assurance
P. 273
272 10. Research methods in flow assurance
hydrogen bonded if the distance between oxygens was less then 0.35 nm and the hydrogen
bond (O-H-----O) angle was not <145°. These values differ from the arbitrary default values
of 0.285 nm and 120° and were selected as to accommodate two criteria discussed in each of
the following paragraphs.
The first criterion is to match the number of hydrogen bonds in real water at a temperature
of 298 K. The value from the literature is 89% of unbroken hydrogen bonds (Makogon, 1974,
1981) at this temperature. The number of nearest neighbors reported by Jorgensen et al. (1983) is
3.54 for the SPC model at these conditions. This can be translated into 88.5% hydrogen bonding
because a 100% hydrogen bonded water molecule (like in the ice lattice) has 4 nearest neighbors
or 4 hydrogen bonds. Results of the SYBYL® simulation at the same temperature with scaling
of the dielectric function produced the average value of 83.1% hydrogen bonding. The number
of hydrogen bonds oscillated during the run to within ±4% of the average value.
The second criterion in selecting hydrogen bond parameters is to reduce the number of
hydrogen bonded trigons which are configurationally and energetically unfavorable. A study
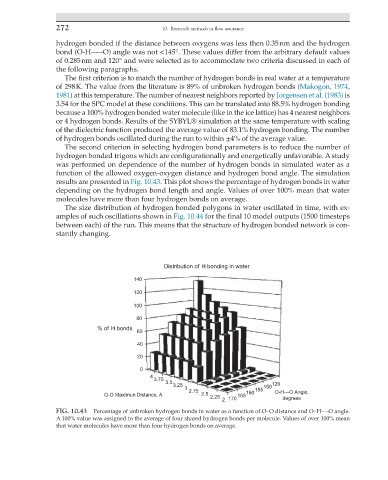
was performed on dependence of the number of hydrogen bonds in simulated water as a
function of the allowed oxygen-oxygen distance and hydrogen bond angle. The simulation
results are presented in Fig. 10.43. This plot shows the percentage of hydrogen bonds in water
depending on the hydrogen bond length and angle. Values of over 100% mean that water
molecules have more than four hydrogen bonds on average.
The size distribution of hydrogen bonded polygons in water oscillated in time, with ex-
amples of such oscillations shown in Fig. 10.44 for the final 10 model outputs (1500 timesteps
between each) of the run. This means that the structure of hydrogen bonded network is con-
stantly changing.
Distribution of H bonding in water
140
120
100
80
% of H bonds
60
40
20
0
4
3.75
3.5 120
3.25 150
3 2.75 155
O-O Maximun Distance, A 2.5 2.25 2 170 165 160 O-H---O Angle,
degrees
FIG. 10.43 Percentage of unbroken hydrogen bonds in water as a function of OO distance and OH O angle.
A 100% value was assigned to the average of four shared hydrogen bonds per molecule. Values of over 100% mean
that water molecules have more than four hydrogen bonds on average.