Page 274 - Handbook Of Multiphase Flow Assurance
P. 274
Molecular modeling 273
Number of hydrogen
bonded rings
140 6
-
120
100 5
7
8
80
9
60 10
40
4
20
0
1 2 3 4 5 6 7 8 9 10
Time (90+n) ps
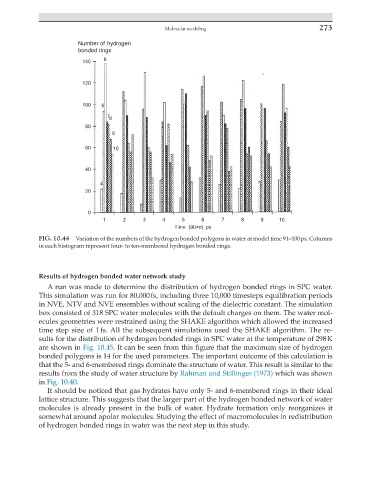
FIG. 10.44 Variation of the numbers of the hydrogen bonded polygons in water at model time 91–100 ps. Columns
in each histogram represent four- to ten-membered hydrogen bonded rings.
Results of hydrogen bonded water network study
A run was made to determine the distribution of hydrogen bonded rings in SPC water.
This simulation was run for 80,000 fs, including three 10,000 timesteps equilibration periods
in NVE, NTV and NVE ensembles without scaling of the dielectric constant. The simulation
box consisted of 318 SPC water molecules with the default charges on them. The water mol-
ecules geometries were restrained using the SHAKE algorithm which allowed the increased
time step size of 1 fs. All the subsequent simulations used the SHAKE algorithm. The re-
sults for the distribution of hydrogen bonded rings in SPC water at the temperature of 298 K
are shown in Fig. 10.45. It can be seen from this figure that the maximum size of hydrogen
bonded polygons is 14 for the used parameters. The important outcome of this calculation is
that the 5- and 6-membered rings dominate the structure of water. This result is similar to the
results from the study of water structure by Rahman and Stillinger (1973) which was shown
in Fig. 10.40.
It should be noticed that gas hydrates have only 5- and 6-membered rings in their ideal
lattice structure. This suggests that the larger part of the hydrogen bonded network of water
molecules is already present in the bulk of water. Hydrate formation only reorganizes it
somewhat around apolar molecules. Studying the effect of macromolecules in redistribution
of hydrogen bonded rings in water was the next step in this study.