Page 301 - Handbook Of Multiphase Flow Assurance
P. 301
300 10. Research methods in flow assurance
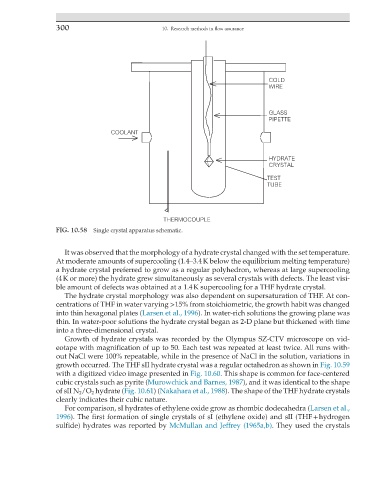
FIG. 10.58 Single crystal apparatus schematic.
It was observed that the morphology of a hydrate crystal changed with the set temperature.
At moderate amounts of supercooling (1.4–3.4 K below the equilibrium melting temperature)
a hydrate crystal preferred to grow as a regular polyhedron, whereas at large supercooling
(4 K or more) the hydrate grew simultaneously as several crystals with defects. The least visi-
ble amount of defects was obtained at a 1.4 K supercooling for a THF hydrate crystal.
The hydrate crystal morphology was also dependent on supersaturation of THF. At con-
centrations of THF in water varying >15% from stoichiometric, the growth habit was changed
into thin hexagonal plates (Larsen et al., 1996). In water-rich solutions the growing plane was
thin. In water-poor solutions the hydrate crystal began as 2-D plane but thickened with time
into a three-dimensional crystal.
Growth of hydrate crystals was recorded by the Olympus SZ-CTV microscope on vid-
eotape with magnification of up to 50. Each test was repeated at least twice. All runs with-
out NaCl were 100% repeatable, while in the presence of NaCl in the solution, variations in
growth occurred. The THF sII hydrate crystal was a regular octahedron as shown in Fig. 10.59
with a digitized video image presented in Fig. 10.60. This shape is common for face-centered
cubic crystals such as pyrite (Murowchick and Barnes, 1987), and it was identical to the shape
of sII N 2 /O 2 hydrate (Fig. 10.61) (Nakahara et al., 1988). The shape of the THF hydrate crystals
clearly indicates their cubic nature.
For comparison, sI hydrates of ethylene oxide grow as rhombic dodecahedra (Larsen et al.,
1996). The first formation of single crystals of sI (ethylene oxide) and sII (THF + hydrogen
sulfide) hydrates was reported by McMullan and Jeffrey (1965a,b). They used the crystals