Page 304 - Handbook Of Multiphase Flow Assurance
P. 304
Experimental study of hydrate crystal growth 303
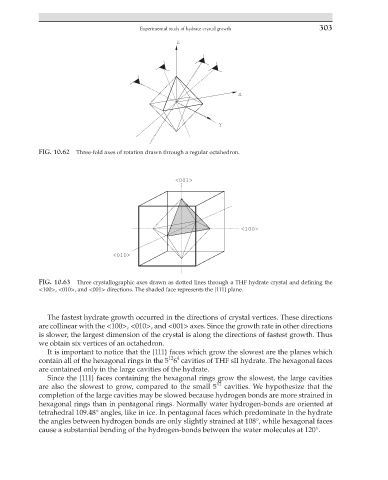
FIG. 10.62 Three-fold axes of rotation drawn through a regular octahedron.
FIG. 10.63 Three crystallographic axes drawn as dotted lines through a THF hydrate crystal and defining the
<100>, <010>, and <001> directions. The shaded face represents the {111} plane.
The fastest hydrate growth occurred in the directions of crystal vertices. These directions
are collinear with the <100>, <010>, and <001> axes. Since the growth rate in other directions
is slower, the largest dimension of the crystal is along the directions of fastest growth. Thus
we obtain six vertices of an octahedron.
It is important to notice that the {111} faces which grow the slowest are the planes which
12 4
contain all of the hexagonal rings in the 5 6 cavities of THF sII hydrate. The hexagonal faces
are contained only in the large cavities of the hydrate.
Since the {111} faces containing the hexagonal rings grow the slowest, the large cavities
12
are also the slowest to grow, compared to the small 5 cavities. We hypothesize that the
completion of the large cavities may be slowed because hydrogen bonds are more strained in
hexagonal rings than in pentagonal rings. Normally water hydrogen-bonds are oriented at
tetrahedral 109.48° angles, like in ice. In pentagonal faces which predominate in the hydrate
the angles between hydrogen bonds are only slightly strained at 108°, while hexagonal faces
cause a substantial bending of the hydrogen-bonds between the water molecules at 120°.