Page 310 - Handbook Of Multiphase Flow Assurance
P. 310
Experimental study of hydrate crystal growth 309
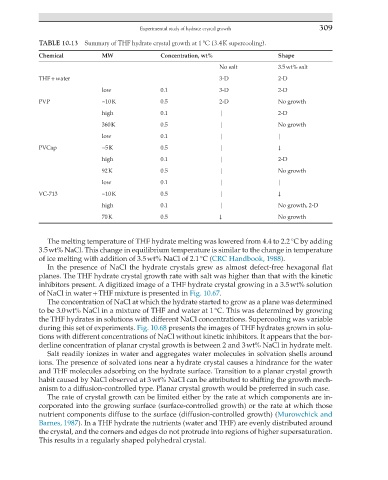
TABLE 10.13 Summary of THF hydrate crystal growth at 1 °C (3.4 K supercooling).
Chemical MW Concentration, wt% Shape
No salt 3.5 wt% salt
THF + water 3-D 2-D
low 0.1 3-D 2-D
PVP ~10 K 0.5 2-D No growth
high 0.1 | 2-D
360 K 0.5 | No growth
low 0.1 | |
PVCap ~5 K 0.5 | ↓
high 0.1 | 2-D
92 K 0.5 | No growth
low 0.1 | |
VC-713 ~10 K 0.5 | ↓
high 0.1 | No growth, 2-D
70 K 0.5 ↓ No growth
The melting temperature of THF hydrate melting was lowered from 4.4 to 2.2 °C by adding
3.5 wt% NaCl. This change in equilibrium temperature is similar to the change in temperature
of ice melting with addition of 3.5 wt% NaCl of 2.1 °C (CRC Handbook, 1988).
In the presence of NaCl the hydrate crystals grew as almost defect-free hexagonal flat
planes. The THF hydrate crystal growth rate with salt was higher than that with the kinetic
inhibitors present. A digitized image of a THF hydrate crystal growing in a 3.5 wt% solution
of NaCl in water + THF mixture is presented in Fig. 10.67.
The concentration of NaCl at which the hydrate started to grow as a plane was determined
to be 3.0 wt% NaCl in a mixture of THF and water at 1 °C. This was determined by growing
the THF hydrates in solutions with different NaCl concentrations. Supercooling was variable
during this set of experiments. Fig. 10.68 presents the images of THF hydrates grown in solu-
tions with different concentrations of NaCl without kinetic inhibitors. It appears that the bor-
derline concentration of planar crystal growth is between 2 and 3 wt% NaCl in hydrate melt.
Salt readily ionizes in water and aggregates water molecules in solvation shells around
ions. The presence of solvated ions near a hydrate crystal causes a hindrance for the water
and THF molecules adsorbing on the hydrate surface. Transition to a planar crystal growth
habit caused by NaCl observed at 3 wt% NaCl can be attributed to shifting the growth mech-
anism to a diffusion-controlled type. Planar crystal growth would be preferred in such case.
The rate of crystal growth can be limited either by the rate at which components are in-
corporated into the growing surface (surface-controlled growth) or the rate at which those
nutrient components diffuse to the surface (diffusion-controlled growth) (Murowchick and
Barnes, 1987). In a THF hydrate the nutrients (water and THF) are evenly distributed around
the crystal, and the corners and edges do not protrude into regions of higher supersaturation.
This results in a regularly shaped polyhedral crystal.