Page 315 - Handbook Of Multiphase Flow Assurance
P. 315
314 10. Research methods in flow assurance
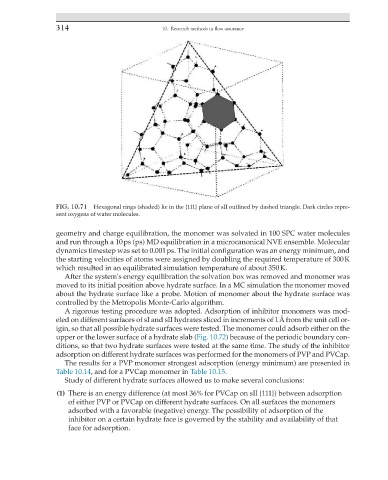
FIG. 10.71 Hexagonal rings (shaded) lie in the {111} plane of sII outlined by dashed triangle. Dark circles repre-
sent oxygens of water molecules.
geometry and charge equilibration, the monomer was solvated in 100 SPC water molecules
and run through a 10 ps (ps) MD equilibration in a microcanonical NVE ensemble. Molecular
dynamics timestep was set to 0.001 ps. The initial configuration was an energy minimum, and
the starting velocities of atoms were assigned by doubling the required temperature of 300 K
which resulted in an equilibrated simulation temperature of about 350 K.
After the system's energy equilibration the solvation box was removed and monomer was
moved to its initial position above hydrate surface. In a MC simulation the monomer moved
about the hydrate surface like a probe. Motion of monomer about the hydrate surface was
controlled by the Metropolis Monte-Carlo algorithm.
A rigorous testing procedure was adopted. Adsorption of inhibitor monomers was mod-
eled on different surfaces of sI and sII hydrates sliced in increments of 1 Å from the unit cell or-
igin, so that all possible hydrate surfaces were tested. The monomer could adsorb either on the
upper or the lower surface of a hydrate slab (Fig. 10.72) because of the periodic boundary con-
ditions, so that two hydrate surfaces were tested at the same time. The study of the inhibitor
adsorption on different hydrate surfaces was performed for the monomers of PVP and PVCap.
The results for a PVP monomer strongest adsorption (energy minimum) are presented in
Table 10.14, and for a PVCap monomer in Table 10.15.
Study of different hydrate surfaces allowed us to make several conclusions:
(1) There is an energy difference (at most 36% for PVCap on sII {111}) between adsorption
of either PVP or PVCap on different hydrate surfaces. On all surfaces the monomers
adsorbed with a favorable (negative) energy. The possibility of adsorption of the
inhibitor on a certain hydrate face is governed by the stability and availability of that
face for adsorption.