Page 320 - Handbook Of Multiphase Flow Assurance
P. 320
Computer modeling of interaction between a hydrate surface and an inhibitor 319
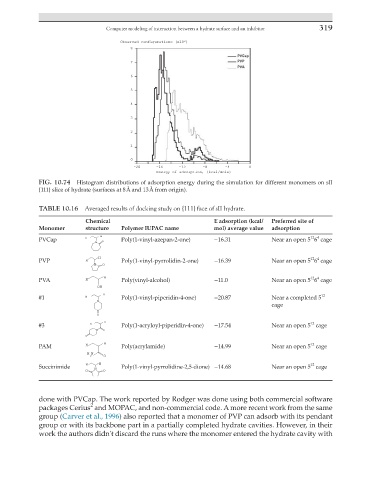
FIG. 10.74 Histogram distributions of adsorption energy during the simulation for different monomers on sII
{111} slice of hydrate (surfaces at 8 Å and 13 Å from origin).
TABLE 10.16 Averaged results of docking study on {111} face of sII hydrate.
Chemical E adsorption (kcal/ Preferred site of
Monomer structure Polymer IUPAC name mol) average value adsorption
12 4
PVCap Poly(1-vinyl-azepan-2-one) −16.31 Near an open 5 6 cage
12 4
PVP Poly(1-vinyl-pyrrolidin-2-one) −16.39 Near an open 5 6 cage
12 4
PVA Poly(vinyl-alcohol) −11.0 Near an open 5 6 cage
12
#1 Poly(1-vinyl-piperidin-4-one) −20.87 Near a completed 5
cage
12
#3 Poly(1-acryloyl-piperidin-4-one) −17.54 Near an open 5 cage
12
PAM Poly(acrylamide) −14.99 Near an open 5 cage
12
Succinimide Poly(1-vinyl-pyrrolidine-2,5-dione) −14.68 Near an open 5 cage
done with PVCap. The work reported by Rodger was done using both commercial software
2
packages Cerius and MOPAC, and non-commercial code. A more recent work from the same
group (Carver et al., 1996) also reported that a monomer of PVP can adsorb with its pendant
group or with its backbone part in a partially completed hydrate cavities. However, in their
work the authors didn't discard the runs where the monomer entered the hydrate cavity with