Page 325 - Handbook Of Multiphase Flow Assurance
P. 325
324 10. Research methods in flow assurance
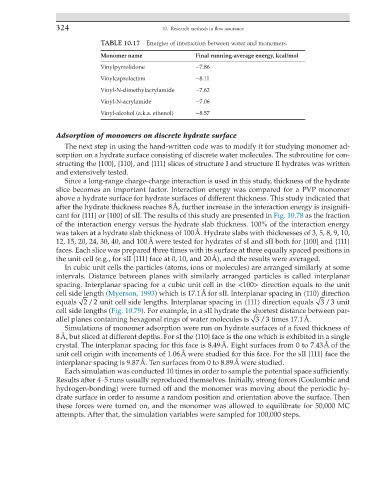
TABLE 10.17 Energies of interaction between water and monomers.
Monomer name Final running-average energy, kcal/mol
Vinylpyrrolidone −7.86
Vinylcaprolactam −8.11
Vinyl-N-dimethylacrylamide −7.62
Vinyl-N-acrylamide −7.06
Vinyl-alcohol (a.k.a. ethenol) −8.57
Adsorption of monomers on discrete hydrate surface
The next step in using the hand-written code was to modify it for studying monomer ad-
sorption on a hydrate surface consisting of discrete water molecules. The subroutine for con-
structing the {100}, {110}, and {111} slices of structure I and structure II hydrates was written
and extensively tested.
Since a long-range charge-charge interaction is used in this study, thickness of the hydrate
slice becomes an important factor. Interaction energy was compared for a PVP monomer
above a hydrate surface for hydrate surfaces of different thickness. This study indicated that
after the hydrate thickness reaches 8 Å, further increase in the interaction energy is insignifi-
cant for {111} or {100} of sII. The results of this study are presented in Fig. 10.78 as the fraction
of the interaction energy versus the hydrate slab thickness. 100% of the interaction energy
was taken at a hydrate slab thickness of 100 Å. Hydrate slabs with thicknesses of 3, 5, 8, 9, 10,
12, 15, 20, 24, 30, 40, and 100 Å were tested for hydrates of sI and sII both for {100} and {111}
faces. Each slice was prepared three times with its surface at three equally spaced positions in
the unit cell (e.g., for sII {111} face at 0, 10, and 20 Å), and the results were averaged.
In cubic unit cells the particles (atoms, ions or molecules) are arranged similarly at some
intervals. Distance between planes with similarly arranged particles is called interplanar
spacing. Interplanar spacing for a cubic unit cell in the <100> direction equals to the unit
cell side length (Myerson, 1993) which is 17.1 Å for sII. Interplanar spacing in 〈110〉 direction
/
/
equals 22 unit cell side lengths. Interplanar spacing in 〈111〉 direction equals 33 unit
cell side lengths (Fig. 10.79). For example, in a sII hydrate the shortest distance between par-
allel planes containing hexagonal rings of water molecules is 33 times 17.1 Å.
/
Simulations of monomer adsorption were run on hydrate surfaces of a fixed thickness of
8 Å, but sliced at different depths. For sI the {110} face is the one which is exhibited in a single
crystal. The interplanar spacing for this face is 8.49 Å. Eight surfaces from 0 to 7.43 Å of the
unit cell origin with increments of 1.06 Å were studied for this face. For the sII {111} face the
interplanar spacing is 9.87 Å. Ten surfaces from 0 to 8.89 Å were studied.
Each simulation was conducted 10 times in order to sample the potential space sufficiently.
Results after 4–5 runs usually reproduced themselves. Initially, strong forces (Coulombic and
hydrogen-bonding) were turned off and the monomer was moving about the periodic hy-
drate surface in order to assume a random position and orientation above the surface. Then
these forces were turned on, and the monomer was allowed to equilibrate for 50,000 MC
attempts. After that, the simulation variables were sampled for 100,000 steps.