Page 327 - Handbook Of Multiphase Flow Assurance
P. 327
326 10. Research methods in flow assurance
In order to avoid the physically unrealistic results the monomer's orientation was restricted
so that it points with its backbone part away from the hydrate surface. The nitrogen-backbone
covalent bond axis was allowed to vary between 0° (normal to surface) and 90° (flat on sur-
face) with respect to the plane of hydrate surface.
The main variables monitored during the simulation of monomers' adsorption on sI and
sII hydrates were: (1) running-average energy, (2) number of hydrogen bonds, and (3) cor-
relation functions between selected monomer atoms and a hydrate surface. It was found that
only on some slices monomer preferred to adsorb into a partially completed cavity. In other
cases the monomer was adsorbed on the surface without entering the cavity. Large cavities
were a preferred adsorption site, compared to the small cavities (Table 10.18).
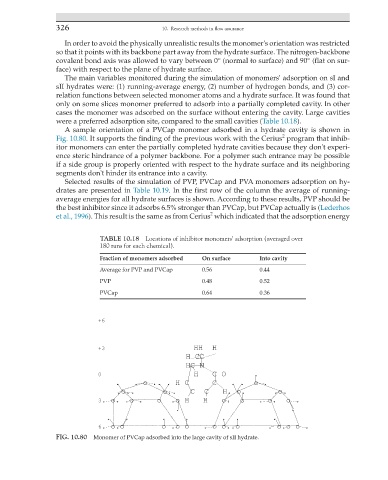
A sample orientation of a PVCap monomer adsorbed in a hydrate cavity is shown in
2
Fig. 10.80. It supports the finding of the previous work with the Cerius program that inhib-
itor monomers can enter the partially completed hydrate cavities because they don't experi-
ence steric hindrance of a polymer backbone. For a polymer such entrance may be possible
if a side group is properly oriented with respect to the hydrate surface and its neighboring
segments don't hinder its entrance into a cavity.
Selected results of the simulation of PVP, PVCap and PVA monomers adsorption on hy-
drates are presented in Table 10.19. In the first row of the column the average of running-
average energies for all hydrate surfaces is shown. According to these results, PVP should be
the best inhibitor since it adsorbs 6.5% stronger than PVCap, but PVCap actually is (Lederhos
2
et al., 1996). This result is the same as from Cerius which indicated that the adsorption energy
TABLE 10.18 Locations of inhibitor monomers' adsorption (averaged over
180 runs for each chemical).
Fraction of monomers adsorbed On surface Into cavity
Average for PVP and PVCap 0.56 0.44
PVP 0.48 0.52
PVCap 0.64 0.36
FIG. 10.80 Monomer of PVCap adsorbed into the large cavity of sII hydrate.