Page 332 - Handbook Of Multiphase Flow Assurance
P. 332
Computer modeling of interaction between a hydrate surface and an inhibitor 331
–4
111
100
110
Surface potential energy per molecule, kcal/mol –10
–6
–8
–12
Large
cavity Small
completed cavity
completed
–14
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17
Distance from the unit cell origin to the surface top, Å
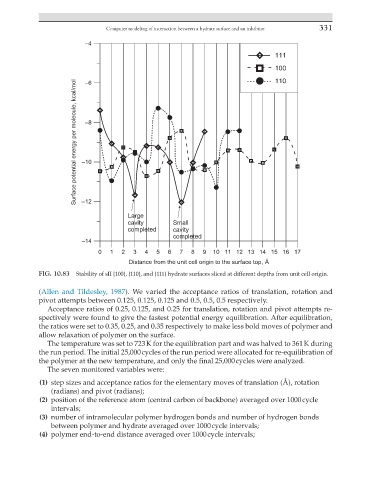
FIG. 10.83 Stability of sII {100}, {110}, and {111} hydrate surfaces sliced at different depths from unit cell origin.
(Allen and Tildesley, 1987). We varied the acceptance ratios of translation, rotation and
pivot attempts between 0.125, 0.125, 0.125 and 0.5, 0.5, 0.5 respectively.
Acceptance ratios of 0.25, 0.125, and 0.25 for translation, rotation and pivot attempts re-
spectively were found to give the fastest potential energy equilibration. After equilibration,
the ratios were set to 0.35, 0.25, and 0.35 respectively to make less bold moves of polymer and
allow relaxation of polymer on the surface.
The temperature was set to 723 K for the equilibration part and was halved to 361 K during
the run period. The initial 25,000 cycles of the run period were allocated for re-equilibration of
the polymer at the new temperature, and only the final 25,000 cycles were analyzed.
The seven monitored variables were:
(1) step sizes and acceptance ratios for the elementary moves of translation (Å), rotation
(radians) and pivot (radians);
(2) position of the reference atom (central carbon of backbone) averaged over 1000 cycle
intervals;
(3) number of intramolecular polymer hydrogen bonds and number of hydrogen bonds
between polymer and hydrate averaged over 1000 cycle intervals;
(4) polymer end-to-end distance averaged over 1000 cycle intervals;