Page 334 - Handbook Of Multiphase Flow Assurance
P. 334
Computer modeling of interaction between a hydrate surface and an inhibitor 333
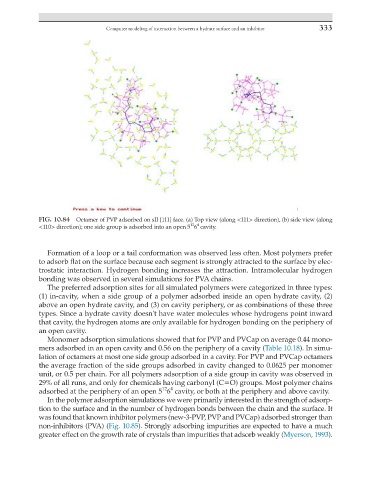
FIG. 10.84 Octamer of PVP adsorbed on sII {111} face. (a) Top view (along <111> direction), (b) side view (along
12 4
<110> direction); one side group is adsorbed into an open 5 6 cavity.
Formation of a loop or a tail conformation was observed less often. Most polymers prefer
to adsorb flat on the surface because each segment is strongly attracted to the surface by elec-
trostatic interaction. Hydrogen bonding increases the attraction. Intramolecular hydrogen
bonding was observed in several simulations for PVA chains.
The preferred adsorption sites for all simulated polymers were categorized in three types:
(1) in-cavity, when a side group of a polymer adsorbed inside an open hydrate cavity, (2)
above an open hydrate cavity, and (3) on cavity periphery, or as combinations of these three
types. Since a hydrate cavity doesn't have water molecules whose hydrogens point inward
that cavity, the hydrogen atoms are only available for hydrogen bonding on the periphery of
an open cavity.
Monomer adsorption simulations showed that for PVP and PVCap on average 0.44 mono-
mers adsorbed in an open cavity and 0.56 on the periphery of a cavity (Table 10.18). In simu-
lation of octamers at most one side group adsorbed in a cavity. For PVP and PVCap octamers
the average fraction of the side groups adsorbed in cavity changed to 0.0625 per monomer
unit, or 0.5 per chain. For all polymers adsorption of a side group in cavity was observed in
29% of all runs, and only for chemicals having carbonyl (CO) groups. Most polymer chains
12 4
adsorbed at the periphery of an open 5 6 cavity, or both at the periphery and above cavity.
In the polymer adsorption simulations we were primarily interested in the strength of adsorp-
tion to the surface and in the number of hydrogen bonds between the chain and the surface. It
was found that known inhibitor polymers (new-3-PVP, PVP and PVCap) adsorbed stronger than
non-inhibitors (PVA) (Fig. 10.85). Strongly adsorbing impurities are expected to have a much
greater effect on the growth rate of crystals than impurities that adsorb weakly (Myerson, 1993).