Page 329 - Handbook Of Multiphase Flow Assurance
P. 329
328 10. Research methods in flow assurance
The adsorption energy exhibited by PVCap monomer is weaker than that of PVP, although
PVCap is a better inhibitor than PVP. The weaker adsorption of PVCap monomer can be
explained by the larger hydrocarbon part of its side group compared to PVP and the same
sizes of polar amide groups. Both PVP and PVCap adsorbed to hydrate stronger than a non-
inhibitor PVA.
After averaging the results for all 300 runs for the adsorption of PVP, PVCap, and PVA
monomers on sII {111} surfaces it was found that if a monomer adsorbed to the hydrate sur-
face with two hydrogen bonds (e.g., stronger), it was at a larger distance from surface than
if it were adsorbed with one or no hydrogen bonds (Table 10.20). This suggests that strong
adsorption through hydrogen bonding cannot occur inside a hydrate cavity because there are
no water hydrogens pointing inward the cavity. On the hydrate surface and on periphery of
the cavities the hydrogens pointing away from the surface are present.
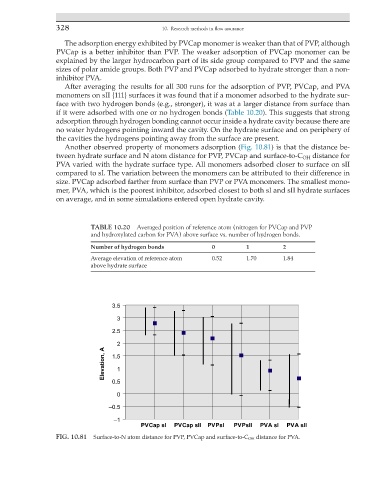
Another observed property of monomers adsorption (Fig. 10.81) is that the distance be-
tween hydrate surface and N atom distance for PVP, PVCap and surface-to-C OH distance for
PVA varied with the hydrate surface type. All monomers adsorbed closer to surface on sII
compared to sI. The variation between the monomers can be attributed to their difference in
size. PVCap adsorbed farther from surface than PVP or PVA monomers. The smallest mono-
mer, PVA, which is the poorest inhibitor, adsorbed closest to both sI and sII hydrate surfaces
on average, and in some simulations entered open hydrate cavity.
TABLE 10.20 Averaged position of reference atom (nitrogen for PVCap and PVP
and hydroxylated carbon for PVA) above surface vs. number of hydrogen bonds.
Number of hydrogen bonds 0 1 2
Average elevation of reference atom 0.52 1.70 1.84
above hydrate surface
3.5
3
2.5
2
Elevation, A 1.5 1
0.5
0
–0.5
–1
PVCap sI PVCap sII PVPsI PVPsII PVA sI PVA sII
FIG. 10.81 Surface-to-N atom distance for PVP, PVCap and surface-to-C OH distance for PVA.