Page 341 - Handbook Of Multiphase Flow Assurance
P. 341
340 10. Research methods in flow assurance
0.80
Fraction of methane adsorbed on hydratesurface
PVA
0.60
PVP
0.40
PVCap
N3PVP
0.20
N2PVP N2PVCap
0.00
40.00 80.00 120.00 160.00 200.00
Monomer size, ų
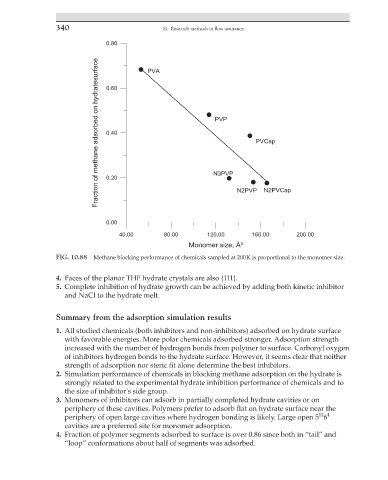
FIG. 10.88 Methane blocking performance of chemicals sampled at 200 K is proportional to the monomer size.
4. Faces of the planar THF hydrate crystals are also {111}.
5. Complete inhibition of hydrate growth can be achieved by adding both kinetic inhibitor
and NaCl to the hydrate melt.
Summary from the adsorption simulation results
1. All studied chemicals (both inhibitors and non-inhibitors) adsorbed on hydrate surface
with favorable energies. More polar chemicals adsorbed stronger. Adsorption strength
increased with the number of hydrogen bonds from polymer to surface. Carbonyl oxygen
of inhibitors hydrogen bonds to the hydrate surface. However, it seems clear that neither
strength of adsorption nor steric fit alone determine the best inhibitors.
2. Simulation performance of chemicals in blocking methane adsorption on the hydrate is
strongly related to the experimental hydrate inhibition performance of chemicals and to
the size of inhibitor's side group.
3. Monomers of inhibitors can adsorb in partially completed hydrate cavities or on
periphery of these cavities. Polymers prefer to adsorb flat on hydrate surface near the
12 4
periphery of open large cavities where hydrogen bonding is likely. Large open 5 6
cavities are a preferred site for monomer adsorption.
4. Fraction of polymer segments adsorbed to surface is over 0.86 since both in “tail” and
“loop” conformations about half of segments was adsorbed.