Page 129 - Handbook of Thermal Analysis of Construction Materials
P. 129
112 Chapter 3 - Formation and Hydration
It reappeared when alkalis were leached out into the aqueous phase. More
than one type of C-S-H appears to have been detected. Small amounts of
MgO were present at three days of hydration and beyond. At one year,
phases identified included C-S-H, monosulfate/C AH , syngenite (only in
4 13
the low alkali cement), brucite, and Ca(OH) .
2
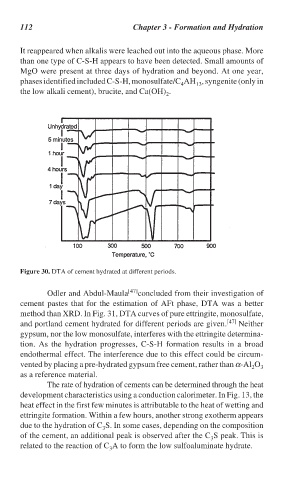
Figure 30. DTA of cement hydrated at different periods.
Odler and Abdul-Maula [47] concluded from their investigation of
cement pastes that for the estimation of AFt phase, DTA was a better
method than XRD. In Fig. 31, DTA curves of pure ettringite, monosulfate,
and portland cement hydrated for different periods are given. [47] Neither
gypsum, nor the low monosulfate, interferes with the ettringite determina-
tion. As the hydration progresses, C-S-H formation results in a broad
endothermal effect. The interference due to this effect could be circum-
vented by placing a pre-hydrated gypsum free cement, rather than α-Al O 3
2
as a reference material.
The rate of hydration of cements can be determined through the heat
development characteristics using a conduction calorimeter. In Fig. 13, the
heat effect in the first few minutes is attributable to the heat of wetting and
ettringite formation. Within a few hours, another strong exotherm appears
due to the hydration of C S. In some cases, depending on the composition
3
of the cement, an additional peak is observed after the C S peak. This is
3
related to the reaction of C A to form the low sulfoaluminate hydrate.
3