Page 231 - Handbook of Thermal Analysis of Construction Materials
P. 231
Section 3.0 - Non-Chloride Accelerators 213
obtained with 1.5–3.0% Ca thiocyanate and 3% Na, K, or Li thiocyanates.
These data imply that the differences in the microstructure are responsible
for the variation in the intrinsic strengths of the pastes when compared at
equal degrees of hydration (equal calcium hydroxide contents).
Applying DSC, Abdelrazig, et al., [23] compared the amount of lime
formed when portland cement was hydrated from 1 hour to 3 days in the
presence of calcium chloride, calcium nitrate, sodium thiocyanate, and
calcium thiocyanate. At 3 hours, all salts acted as accelerators, but calcium
chloride appeared to be the best accelerator. At about 6 hours, more
Ca(OH) was formed in the presence of calcium thiocyanates. At 3 days, the
2
chloride and the thiocyanates produced about the same amount calcium
hydroxide. Calcium nitrate was found to be the least efficient accelerator.
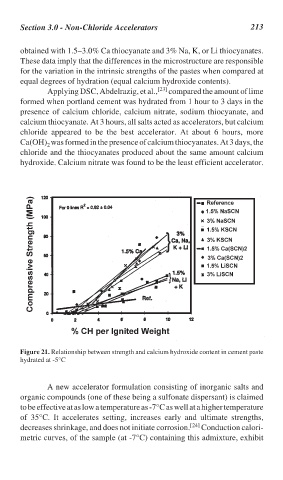
Figure 21. Relationship between strength and calcium hydroxide content in cement paste
hydrated at -5°C
A new accelerator formulation consisting of inorganic salts and
organic compounds (one of these being a sulfonate dispersant) is claimed
to be effective at as low a temperature as -7°C as well at a higher temperature
of 35°C. It accelerates setting, increases early and ultimate strengths,
decreases shrinkage, and does not initiate corrosion. [24] Conduction calori-
metric curves, of the sample (at -7°C) containing this admixture, exhibit