Page 201 - Handbook of Adhesion Promoters
P. 201
194 Selection of Adhesion Promoters for Different
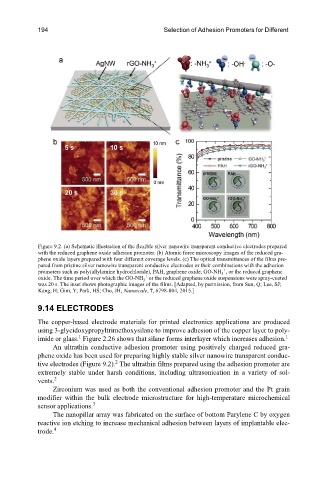
Figure 9.2. (a) Schematic illustration of the flexible silver nanowire transparent conductive electrodes prepared
with the reduced graphene oxide adhesion promoter. (b) Atomic force microscopy images of the reduced gra-
phene oxide layers prepared with four different coverage levels. (c) The optical transmittances of the films pre-
pared from pristine silver nanowire transparent conductive electrodes or their combinations with the adhesion
+
promoters such as poly(allylamine hydrochloride), PAH, graphene oxide, GO-NH , or the reduced graphene
3
+
oxide. The time period over which the GO-NH or the reduced graphene oxide suspensions were spray-coated
3
was 20 s. The inset shows photographic images of the films. [Adapted, by permission, from Sun, Q; Lee, SJ;
Kang, H; Gim, Y; Park, HS; Cho, JH, Nanoscale, 7, 6798-804, 2015.]
9.14 ELECTRODES
The copper-based electrode materials for printed electronics applications are produced
using 3-glycidoxypropyltrimethoxysilane to improve adhesion of the copper layer to poly-
1
1
imide or glass. Figure 2.26 shows that silane forms interlayer which increases adhesion.
An ultrathin conductive adhesion promoter using positively charged reduced gra-
phene oxide has been used for preparing highly stable silver nanowire transparent conduc-
2
tive electrodes (Figure 9.2). The ultrathin films prepared using the adhesion promoter are
extremely stable under harsh conditions, including ultrasonication in a variety of sol-
2
vents.
Zirconium was used as both the conventional adhesion promoter and the Pt grain
modifier within the bulk electrode microstructure for high-temperature microchemical
3
sensor applications.
The nanopillar array was fabricated on the surface of bottom Parylene C by oxygen
reactive ion etching to increase mechanical adhesion between layers of implantable elec-
4
trode.