Page 71 - Handbook of Adhesion Promoters
P. 71
64 Substrates - Surface Condition and Treat-
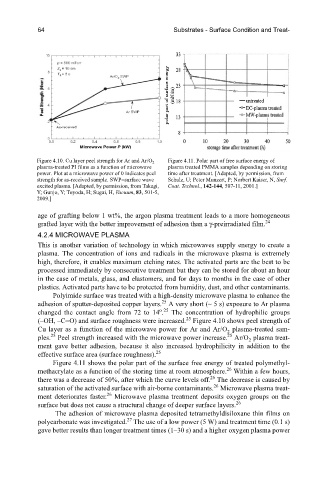
Figure 4.10. Cu layer peel strength for Ar and Ar/O Figure 4.11. Polar part of free surface energy of
2
plasma-treated PI films as a function of microwave plasma treated PMMA samples depending on storing
power. Plot at a microwave power of 0 indicates peel time after treatment. [Adapted, by permission, from
strength for as-received sample. SWP=surface wave Schulz, U; Peter Munzert, P; Norbert Kaiser, N, Surf.
excited plasma. [Adapted, by permission, from Takagi, Coat. Technol., 142-144, 507-11, 2001.]
Y; Gunjo, Y; Toyoda, H; Sugai, H, Vacuum, 83, 501-5,
2009.]
age of grafting below 1 wt%, the argon plasma treatment leads to a more homogeneous
24
grafted layer with the better improvement of adhesion than a γ-preirradiated film.
4.2.4 MICROWAVE PLASMA
This is another variation of technology in which microwaves supply energy to create a
plasma. The concentration of ions and radicals in the microwave plasma is extremely
high, therefore, it enables maximum etching rates. The activated parts are the best to be
processed immediately by consecutive treatment but they can be stored for about an hour
in the case of metals, glass, and elastomers, and for days to months in the case of other
plastics. Activated parts have to be protected from humidity, dust, and other contaminants.
Polyimide surface was treated with a high-density microwave plasma to enhance the
25
adhesion of sputter-deposited copper layers. A very short (~ 5 s) exposure to Ar plasma
o 25
changed the contact angle from 72 to 14 . The concentration of hydrophilic groups
25
(–OH, –C=O) and surface roughness were increased. Figure 4.10 shows peel strength of
Cu layer as a function of the microwave power for Ar and Ar/O plasma-treated sam-
2
25
25
ples. Peel strength increased with the microwave power increase. Ar/O plasma treat-
2
ment gave better adhesion, because it also increased hydrophilicity in addition to the
25
effective surface area (surface roughness).
Figure 4.11 shows the polar part of the surface free energy of treated polymethyl-
26
methacrylate as a function of the storing time at room atmosphere. Within a few hours,
26
there was a decrease of 50%, after which the curve levels off. The decrease is caused by
26
saturation of the activated surface with air-borne contaminants. Microwave plasma treat-
26
ment deteriorates faster. Microwave plasma treatment deposits oxygen groups on the
26
surface but does not cause a structural change of deeper surface layers.
The adhesion of microwave plasma deposited tetramethyldisiloxane thin films on
27
polycarbonate was investigated. The use of a low power (5 W) and treatment time (0.1 s)
gave better results than longer treatment times (1–30 s) and a higher oxygen plasma power