Page 119 - Handbook of Adhesives and Sealants
P. 119
Theories of Adhesion 87
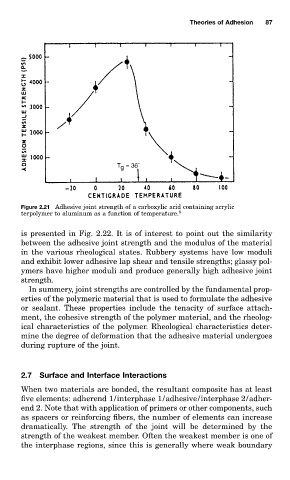
Figure 2.21 Adhesive joint strength of a carboxylic acid containing acrylic
terpolymer to aluminum as a function of temperature. 8
is presented in Fig. 2.22. It is of interest to point out the similarity
between the adhesive joint strength and the modulus of the material
in the various rheological states. Rubbery systems have low moduli
and exhibit lower adhesive lap shear and tensile strengths; glassy pol-
ymers have higher moduli and produce generally high adhesive joint
strength.
In summery, joint strengths are controlled by the fundamental prop-
erties of the polymeric material that is used to formulate the adhesive
or sealant. These properties include the tenacity of surface attach-
ment, the cohesive strength of the polymer material, and the rheolog-
ical characteristics of the polymer. Rheological characteristics deter-
mine the degree of deformation that the adhesive material undergoes
during rupture of the joint.
2.7 Surface and Interface Interactions
When two materials are bonded, the resultant composite has at least
five elements: adherend 1/interphase 1/adhesive/interphase 2/adher-
end 2. Note that with application of primers or other components, such
as spacers or reinforcing fibers, the number of elements can increase
dramatically. The strength of the joint will be determined by the
strength of the weakest member. Often the weakest member is one of
the interphase regions, since this is generally where weak boundary