Page 115 - Handbook of Adhesives and Sealants
P. 115
Theories of Adhesion 83
Favorable surface energetics are a necessary, but not singularly suf-
ficient, condition for bond formation. Proper spreading kinetics and
rheology are also required. The adhesive must have the necessary rhe-
ology to flow on the time scale of the bond formation. Time dependent
flow of the adhesive, which is often confused with or combined with
the energetic ‘‘wetting’’ of the substrate by an adhesive, is a major
factor in application of adhesives. The major property affecting flow is
viscosity and, therefore, molecular weight.
2.6.2.2 Properties affecting cohesion. In addition to good adhesion, an
adhesive or sealant must have satisfactory cohesive strength. Factors
that can affect cohesive strength include molecular weight, crystallin-
ity, hydrogen bonding, crosslinking, and compatibility (in multi-phase
systems).
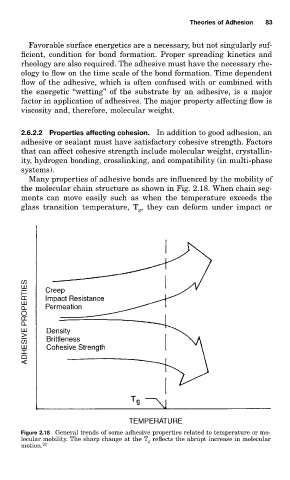
Many properties of adhesive bonds are influenced by the mobility of
the molecular chain structure as shown in Fig. 2.18. When chain seg-
ments can move easily such as when the temperature exceeds the
glass transition temperature, T , they can deform under impact or
g
Figure 2.18 General trends of some adhesive properties related to temperature or mo-
lecular mobility. The sharp change at the T g reflects the abrupt increase in molecular
motion. 21