Page 113 - Handbook of Adhesives and Sealants
P. 113
Theories of Adhesion 81
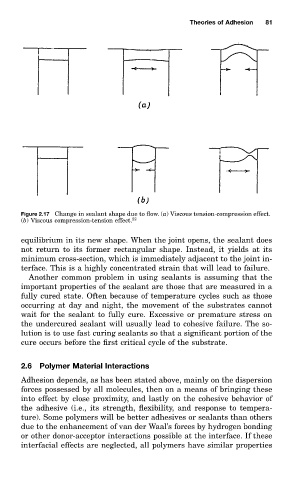
Figure 2.17 Change in sealant shape due to flow. (a) Viscous tension-compression effect.
(b) Viscous compression-tension effect. 22
equilibrium in its new shape. When the joint opens, the sealant does
not return to its former rectangular shape. Instead, it yields at its
minimum cross-section, which is immediately adjacent to the joint in-
terface. This is a highly concentrated strain that will lead to failure.
Another common problem in using sealants is assuming that the
important properties of the sealant are those that are measured in a
fully cured state. Often because of temperature cycles such as those
occurring at day and night, the movement of the substrates cannot
wait for the sealant to fully cure. Excessive or premature stress on
the undercured sealant will usually lead to cohesive failure. The so-
lution is to use fast curing sealants so that a significant portion of the
cure occurs before the first critical cycle of the substrate.
2.6 Polymer Material Interactions
Adhesion depends, as has been stated above, mainly on the dispersion
forces possessed by all molecules, then on a means of bringing these
into effect by close proximity, and lastly on the cohesive behavior of
the adhesive (i.e., its strength, flexibility, and response to tempera-
ture). Some polymers will be better adhesives or sealants than others
due to the enhancement of van der Waal’s forces by hydrogen bonding
or other donor-acceptor interactions possible at the interface. If these
interfacial effects are neglected, all polymers have similar properties