Page 93 - Handbook of Adhesives and Sealants
P. 93
Theories of Adhesion 61
For poor wetting: adhesive C substrate
A simple view of the relationship of wetting and adhesion is pro-
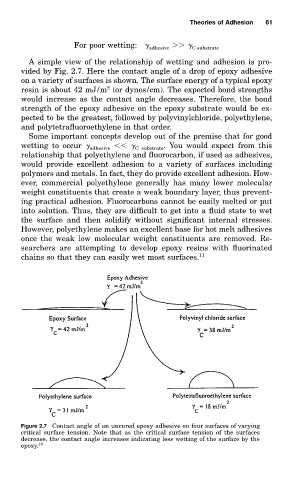
vided by Fig. 2.7. Here the contact angle of a drop of epoxy adhesive
on a variety of surfaces is shown. The surface energy of a typical epoxy
2
resin is about 42 mJ/m (or dynes/cm). The expected bond strengths
would increase as the contact angle decreases. Therefore, the bond
strength of the epoxy adhesive on the epoxy substrate would be ex-
pected to be the greatest, followed by polyvinylchloride, polyethylene,
and polytetrafluoroethylene in that order.
Some important concepts develop out of the premise that for good
wetting to occur adhesive C substrate . You would expect from this
relationship that polyethylene and fluorocarbon, if used as adhesives,
would provide excellent adhesion to a variety of surfaces including
polymers and metals. In fact, they do provide excellent adhesion. How-
ever, commercial polyethylene generally has many lower molecular
weight constituents that create a weak boundary layer, thus prevent-
ing practical adhesion. Fluorocarbons cannot be easily melted or put
into solution. Thus, they are difficult to get into a fluid state to wet
the surface and then solidify without significant internal stresses.
However, polyethylene makes an excellent base for hot melt adhesives
once the weak low molecular weight constituents are removed. Re-
searchers are attempting to develop epoxy resins with fluorinated
chains so that they can easily wet most surfaces. 11
Figure 2.7 Contact angle of an uncured epoxy adhesive on four surfaces of varying
critical surface tension. Note that as the critical surface tension of the surfaces
decrease, the contact angle increases indicating less wetting of the surface by the
epoxy. 10