Page 95 - Handbook of Adhesives and Sealants
P. 95
Theories of Adhesion 63
example the overlap distances of a lap joint, has diminishing returns.
The next chapter will discuss this possibility.
Surface roughness generally aids in adhesive bonding by the me-
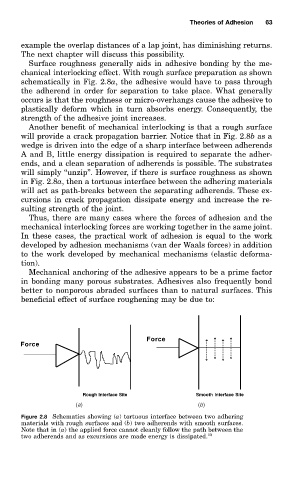
chanical interlocking effect. With rough surface preparation as shown
schematically in Fig. 2.8a, the adhesive would have to pass through
the adherend in order for separation to take place. What generally
occurs is that the roughness or micro-overhangs cause the adhesive to
plastically deform which in turn absorbs energy. Consequently, the
strength of the adhesive joint increases.
Another benefit of mechanical interlocking is that a rough surface
will provide a crack propagation barrier. Notice that in Fig. 2.8b as a
wedge is driven into the edge of a sharp interface between adherends
A and B, little energy dissipation is required to separate the adher-
ends, and a clean separation of adherends is possible. The substrates
will simply ‘‘unzip’’. However, if there is surface roughness as shown
in Fig. 2.8a, then a tortuous interface between the adhering materials
will act as path-breaks between the separating adherends. These ex-
cursions in crack propagation dissipate energy and increase the re-
sulting strength of the joint.
Thus, there are many cases where the forces of adhesion and the
mechanical interlocking forces are working together in the same joint.
In these cases, the practical work of adhesion is equal to the work
developed by adhesion mechanisms (van der Waals forces) in addition
to the work developed by mechanical mechanisms (elastic deforma-
tion).
Mechanical anchoring of the adhesive appears to be a prime factor
in bonding many porous substrates. Adhesives also frequently bond
better to nonporous abraded surfaces than to natural surfaces. This
beneficial effect of surface roughening may be due to:
Force
Force
Rough Interface Site Smooth Interface Site
(a) (b)
Figure 2.8 Schematics showing (a) tortuous interface between two adhering
materials with rough surfaces and (b) two adherends with smooth surfaces.
Note that in (a) the applied force cannot cleanly follow the path between the
two adherends and as excursions are made energy is dissipated. 10