Page 99 - Handbook of Adhesives and Sealants
P. 99
Theories of Adhesion 67
The result of good wetting is simply that there is greater contact area
between adherend and adhesive over which the forces of adhesion
(e.g., van der Waals forces) may act. As shown in the preceding sec-
tions, for maximum wetting, the surface energy of the liquid adhesive
must be less than that of the solid adherend or LV . Table 2.2
C
provides surface tensions for common adhesive liquids and critical sur-
face tension for various solids.
The wetting of surfaces by adhesives can be described by two activ-
ities: a lateral spreading of the film; and a penetration of the fluid
adhesive into the surface cavities that are characteristic of the inher-
ent surface roughness. The first activity is controlled by the relative
surface energies of the adhesive and substrate. The second activity is
controlled mainly by the viscosity of the adhesive and the time it is
in the liquid state.
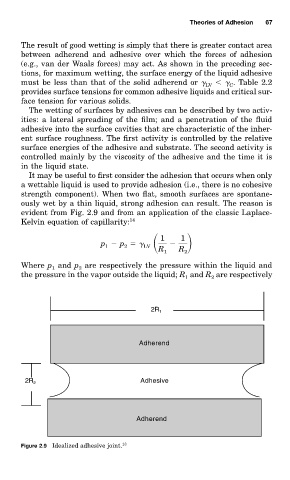
It may be useful to first consider the adhesion that occurs when only
a wettable liquid is used to provide adhesion (i.e., there is no cohesive
strength component). When two flat, smooth surfaces are spontane-
ously wet by a thin liquid, strong adhesion can result. The reason is
evident from Fig. 2.9 and from an application of the classic Laplace-
Kelvin equation of capillarity: 14
1 1
p p LV
2
1
R 1 R 2
Where p and p are respectively the pressure within the liquid and
1
2
the pressure in the vapor outside the liquid; R and R are respectively
2
1
2R 1
Adherend
Adhesive
2R 2
Adherend
Figure 2.9 Idealized adhesive joint. 13