Page 103 - Handbook of Adhesives and Sealants
P. 103
Theories of Adhesion 71
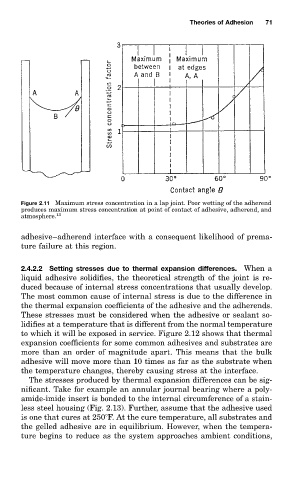
Figure 2.11 Maximum stress concentration in a lap joint. Poor wetting of the adherend
produces maximum stress concentration at point of contact of adhesive, adherend, and
atmosphere. 13
adhesive–adherend interface with a consequent likelihood of prema-
ture failure at this region.
2.4.2.2 Setting stresses due to thermal expansion differences. When a
liquid adhesive solidifies, the theoretical strength of the joint is re-
duced because of internal stress concentrations that usually develop.
The most common cause of internal stress is due to the difference in
the thermal expansion coefficients of the adhesive and the adherends.
These stresses must be considered when the adhesive or sealant so-
lidifies at a temperature that is different from the normal temperature
to which it will be exposed in service. Figure 2.12 shows that thermal
expansion coefficients for some common adhesives and substrates are
more than an order of magnitude apart. This means that the bulk
adhesive will move more than 10 times as far as the substrate when
the temperature changes, thereby causing stress at the interface.
The stresses produced by thermal expansion differences can be sig-
nificant. Take for example an annular journal bearing where a poly-
amide-imide insert is bonded to the internal circumference of a stain-
less steel housing (Fig. 2.13). Further, assume that the adhesive used
is one that cures at 250 F. At the cure temperature, all substrates and
the gelled adhesive are in equilibrium. However, when the tempera-
ture begins to reduce as the system approaches ambient conditions,