Page 124 - Handbook of Battery Materials
P. 124
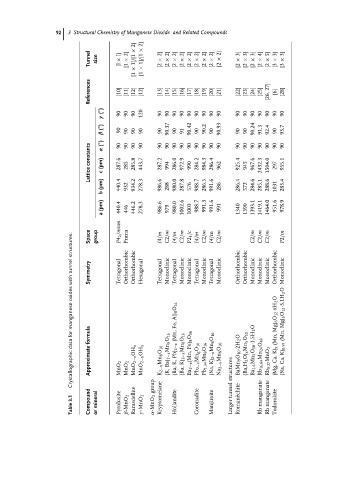
92 3 Structural Chemistry of Manganese Dioxide and Related Compounds
× 2] × 2]
Tunnel size l] [l × 2] × 1]/[1 × 1]/[1 2] [2 × 2] [2 × 2] [2 × 2] [2 × 2] [2 × 2] [2 × 2] [2 × 2] [2 × × 2] [2 3] [2 × 3] [2 × 3] [2 × 4] [2 × 5] [2 × 3] [3 × 3] [3 ×
[1
[1 × [1
References [10] [11] [12] [12] [13] [14] [15] [16] [17] [18] [19] [20] [21] [22] [23] [24] [25] 27] [26, [6] [28]
( ◦ ) 90 90 90 120 90 90 90 90 90 90 90 90 90 90 90 90 90 90 90 90
γ
( ◦ ) 90.37 90.42 90.2 90.93 90.24 91.3 92.4 93.7
β ( ◦ ) 90 90 90 90 90 90 91 90 90 90 90 90
constants α 90 287.6 90 90 285.8 90 443.7 90 287.2 90 90 286.0 90 972.9 90 90 284.2 90 984.3 90 286.4 90 90 925.4 90 90 967.6 90 90 90 90 955.1
Lattice c (pm) 285 994 990 962 945 2432.3 1504.0 297
b (pm) 440.4 932 934.2 278.3 986.6 288 980.0 287.8 576 988.7 286.5 991.6 286 286.4 572 284.6 285.1 288.6 1031 283.4
a (pm) 440.4 P4 2 /mnm 446 446.2 278.3 986.6 979 980.0 1002.6 1003 988.7 991.3 991.6 991 1340 1390 1393.1 1419.1 1464.0 951.6 978.9
with tunnel structures. Space Symmetry group Tetragonal Pnma Orthorhombic Orthorhombic Hexagonal I4/m Tetragonal C2/m Monoclinic I4/m Tetragonal C2/m Monoclinic P2 1 /c Monoclinic I4/m Tetragonal C2/m Monoclinic I4/m Tetragonal C2/m Monoclinic Orthorhombic Orthorhombic C2/m Monoclinic C2/m Monoclinic C2/m Monoclinic Orthorhombic P2/m Monoc
oxides
manganese Al) 8 O 16 (Mn, Fe, Mg) 6 O 12 ·xH 2 O Mg) 6 O 12 ·3.1H 2 O (Mn,
Crystallographic data for Approximate formula MnO 2 MnO 2 MnO 2−x OH x MnO 2−x OH x K 2−x Mn 8 O 16 (K, Ba) 2−x Mn 8 O 16 (Ba, K, Pb) 2−x (Ba, K) 2−x Mn 8 O 16 Fe) 8 O 16 Ba 2−x (Mn, Pb 2−x Mn g O 16 Pb 1.34 Mn 8 O 16 (Na, K) 2−x Mn 8 O 16 Na 2−x Mn 8 O 16 structures BaMn 9 O 10 ·2H 2 O (Ba,H 2 O) x Mn 5 O 10 Ba 1.6 Mn 5 O 10 ·1.5H 2 O Rb 16.6
Table 3.1 Compound or mineral Pyrolusite β-MnO 2 Ramsdellite γ -MnO 2 group α-MnO 2 Kryptomelane Hollandite Coronadite Manjiroite Larger tunnel echite Roman` Rb manganate Rb manganate Todorokite