Page 128 - Handbook of Battery Materials
P. 128
96 3 Structural Chemistry of Manganese Dioxide and Related Compounds
1) The manganese atoms are distributed in a more or less ordered manner at
the slightly distorted octahedral voids in the hexagonally close-packed oxygen
atoms (as described above).
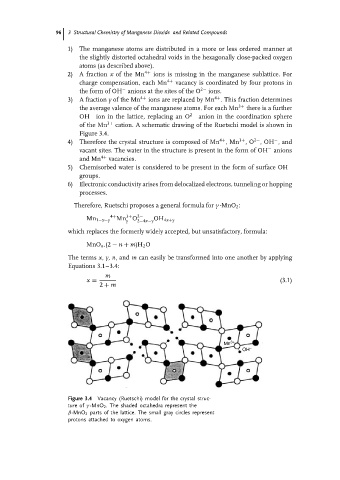
2) A fraction x of the Mn 4+ ions is missing in the manganese sublattice. For
charge compensation, each Mn 4+ vacancy is coordinated by four protons in
the form of OH anions at the sites of the O 2− ions.
−
3) A fraction y of the Mn 4+ ions are replaced by Mn . This fraction determines
4+
the average valence of the manganese atoms. For each Mn 3+ there is a further
OH − ion in the lattice, replacing an O 2− anion in the coordination sphere
of the Mn 3+ cation. A schematic drawing of the Ruetschi model is shown in
Figure 3.4.
3+
4+
4) Therefore the crystal structure is composed of Mn ,Mn ,O ,OH , and
−
2−
vacant sites. The water in the structure is present in the form of OH anions
−
and Mn 4+ vacancies.
5) Chemisorbed water is considered to be present in the form of surface OH −
groups.
6) Electronic conductivity arises from delocalized electrons, tunneling or hopping
processes.
Therefore, Ruetschi proposes a general formula for γ -MnO 2 :
4+ 3+ 2−
Mn 1−x−y Mn O 2−4x−y OH 4x+y
y
which replaces the formerly widely accepted, but unsatisfactory, formula:
MnO n .(2 − n + m)H 2 O
The terms x, y, n,and m can easily be transformed into one another by applying
Equations 3.1–3.4:
m
x = (3.1)
2 + m
3+
Mn
OH −
Figure 3.4 Vacancy (Ruetschi) model for the crystal struc-
ture of γ -MnO 2 . The shaded octahedra represent the
β-MnO 2 parts of the lattice. The small gray circles represent
protons attached to oxygen atoms.