Page 126 - Handbook of Battery Materials
P. 126
94 3 Structural Chemistry of Manganese Dioxide and Related Compounds
broadening made the structure determination very complicated. Additionally, a
large number of significantly different patterns could be observed, depending
strongly on the preparation conditions. Some XRD patterns resembled the diffrac-
tograms of pyrolusite, others were similar to the line-rich patterns of ramsdellite
samples, and many showed only a few broad peaks, that could be indexed on
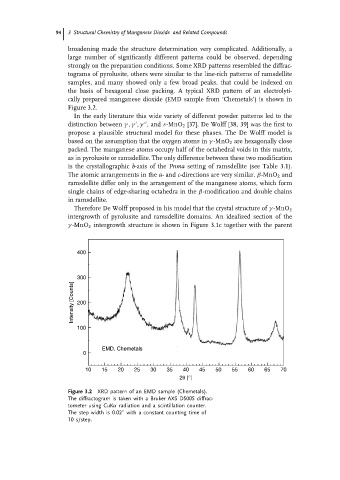
the basis of hexagonal close packing. A typical XRD pattern of an electrolyti-
cally prepared manganese dioxide (EMD sample from ‘Chemetals’) is shown in
Figure 3.2.
In the early literature this wide variety of different powder patterns led to the
distinction between γ , γ , γ ,and ε-MnO 2 [37]. De Wolff [38, 39] was the first to
propose a plausible structural model for these phases. The De Wolff model is
based on the assumption that the oxygen atoms in γ -MnO 2 are hexagonally close
packed. The manganese atoms occupy half of the octahedral voids in this matrix,
as in pyrolusite or ramsdellite. The only difference between these two modification
is the crystallographic b-axis of the Pnma setting of ramsdellite (see Table 3.1).
The atomic arrangements in the a- and c-directions are very similar. β-MnO 2 and
ramsdellite differ only in the arrangement of the manganese atoms, which form
single chains of edge-sharing octahedra in the β-modification and double chains
in ramsdellite.
Therefore De Wolff proposed in his model that the crystal structure of γ -MnO 2
intergrowth of pyrolusite and ramsdellite domains. An idealized section of the
γ -MnO 2 intergrowth structure is shown in Figure 3.1c together with the parent
400
300
Intensity [Counts] 200
100
EMD, Chemetals
0
10 15 20 25 30 35 40 45 50 55 60 65 70
2θ [°]
Figure 3.2 XRD pattern of an EMD sample (Chemetals).
The diffractogram is taken with a Bruker AXS D5005 diffrac-
tometer using CuKα radiation and a scintillation counter.
◦
The step width is 0.02 with a constant counting time of
10 s/step.