Page 217 - Handbook of Battery Materials
P. 217
186 6 Lead Oxides
3
2
1
5
4
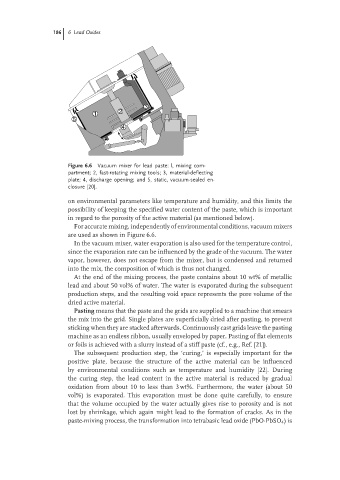
Figure 6.6 Vacuum mixer for lead paste: l, mixing com-
partment; 2, fast-rotating mixing tools; 3, material-deflecting
plate; 4, discharge opening; and 5, static, vacuum-sealed en-
closure [20].
on environmental parameters like temperature and humidity, and this limits the
possibility of keeping the specified water content of the paste, which is important
in regard to the porosity of the active material (as mentioned below).
For accurate mixing, independently of environmental conditions, vacuum mixers
are used as shown in Figure 6.6.
In the vacuum mixer, water evaporation is also used for the temperature control,
since the evaporation rate can be influenced by the grade of the vacuum. The water
vapor, however, does not escape from the mixer, but is condensed and returned
into the mix, the composition of which is thus not changed.
At the end of the mixing process, the paste contains about 10 wt% of metallic
lead and about 50 vol% of water. The water is evaporated during the subsequent
production steps, and the resulting void space represents the pore volume of the
dried active material.
Pasting means that the paste and the grids are supplied to a machine that smears
the mix into the grid. Single plates are superficially dried after pasting, to prevent
sticking when they are stacked afterwards. Continuously cast grids leave the pasting
machine as an endless ribbon, usually enveloped by paper. Pasting of flat elements
or foils is achieved with a slurry instead of a stiff paste (cf., e.g., Ref. [21]).
The subsequent production step, the ‘curing,’ is especially important for the
positive plate, because the structure of the active material can be influenced
by environmental conditions such as temperature and humidity [22]. During
the curing step, the lead content in the active material is reduced by gradual
oxidation from about 10 to less than 3 wt%. Furthermore, the water (about 50
vol%) is evaporated. This evaporation must be done quite carefully, to ensure
that the volume occupied by the water actually gives rise to porosity and is not
lost by shrinkage, which again might lead to the formation of cracks. As in the
paste-mixing process, the transformation into tetrabasic lead oxide (PbO·PbSO 4 )is