Page 397 - Handbook of Battery Materials
P. 397
368 12 Lithium Intercalation Cathode Materials for Lithium-Ion Batteries
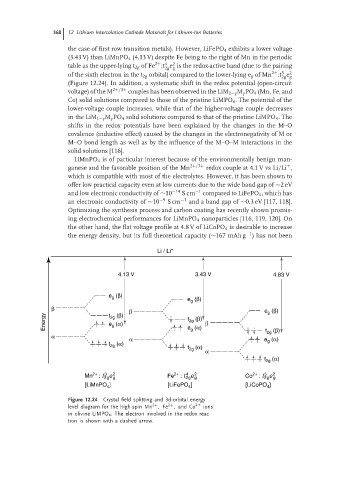
the case of first row transition metals). However, LiFePO 4 exhibits a lower voltage
(3.43 V) than LiMnPO 4 (4.13 V) despite Fe being to the right of Mn in the periodic
2
2+ 4
table as the upper-lying t 2g of Fe :t e is the redox-active band (due to the pairing
2g g
2+ 3
of the sixth electron in the t 2g orbital) compared to the lower-lying e g of Mn :t e 2
2g g
(Figure 12.24). In addition, a systematic shift in the redox potential (open-circuit
voltage) of the M 2+/3+ couples has been observed in the LiM 1−y M y PO 4 (Mn, Fe, and
Co) solid solutions compared to those of the pristine LiMPO 4 . The potential of the
lower-voltage couple increases, while that of the higher-voltage couple decreases
in the LiM 1−y M y PO 4 solid solutions compared to that of the pristine LiMPO 4 .The
shifts in the redox potentials have been explained by the changes in the M–O
covalence (inductive effect) caused by the changes in the electronegativity of M or
M–O bond length as well as by the influence of the M–O–M interactions in the
solid solutions [116].
LiMnPO 4 is of particular interest because of the environmentally benign man-
ganese and the favorable position of the Mn 2+/3+ redox couple at 4.1 V vs Li/Li ,
+
which is compatible with most of the electrolytes. However, it has been shown to
offer low practical capacity even at low currents due to the wide band gap of ∼2eV
and low electronic conductivity of ∼10 −14 Scm −1 compared to LiFePO 4 , which has
an electronic conductivity of ∼10 −9 Scm −1 and a band gap of ∼0.3 eV [117, 118].
Optimizing the synthesis process and carbon coating has recently shown promis-
ing electrochemical performances for LiMnPO 4 nanoparticles [116, 119, 120]. On
the other hand, the flat voltage profile at 4.8 V of LiCoPO 4 is desirable to increase
−1
the energy density, but its full theoretical capacity (∼167 mAh g ) has not been
Li / Li +
4.13 V 3.43 V 4.83 V
e (β)
g
e g (β)
β β e g (β)
Energy t 2g (β) t 2g (β) β
e (α)
g
e g (α)
t 2g (β)
α α e g (α)
t (α) t (α) α
2g
2g
t (α)
2g
2+ 3 2 2+ 4 2 2+ 5 2
2g
2g
Mn : t e g Fe : t e g Co : t e g
2g
[LiMnPO ] [LiFePO ] [LiCoPO ]
4
4
4
Figure 12.24 Crystal field splitting and 3d-orbital energy
level diagram for the high-spin Mn ,Fe ,and Co 2+ ions
2+
2+
in olivine LiMPO 4 . The electron involved in the redox reac-
tion is shown with a dashed arrow.