Page 395 - Handbook of Battery Materials
P. 395
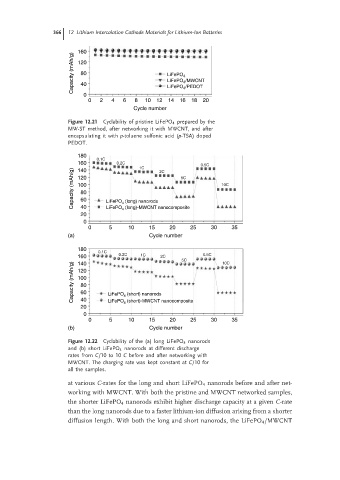
366 12 Lithium Intercalation Cathode Materials for Lithium-Ion Batteries
160
Capacity (mAh/g) 80 LiFePO 4
120
LiFePO 4 /MWCNT
40
0 LiFePO 4 /PEDOT
0 2 4 6 8 10 12 14 16 18 20
Cycle number
Figure 12.21 Cyclability of pristine LiFePO 4 prepared by the
MW-ST method, after networking it with MWCNT, and after
encapsulating it with p-toluene sulfonic acid (p-TSA) doped
PEDOT.
180
0.1C
160 0.2C 1C 2C 0.5C
140
Capacity (mAh/g) 120 LiFePO 4 (long) nanorods 5C 10C
100
80
60
40
20 LiFePO 4 (long)-MWCNT nanocomposite
0
0 5 10 15 20 25 30 35
(a) Cycle number
180
0.1C
160 0.2C 1C 2C 0.5C
5C 10C
Capacity (mAh/g) 100 LiFePO 4 (short) nanorods
140
120
80
60
40
20 LiFePO 4 (short)-MWCNT nanocomposite
0
0 5 10 15 20 25 30 35
(b) Cycle number
Figure 12.22 Cyclability of the (a) long LiFePO 4 nanorods
and (b) short LiFePO 4 nanorods at different discharge
rates from C/10 to 10 C before and after networking with
MWCNT. The charging rate was kept constant at C/10 for
all the samples.
at various C-rates for the long and short LiFePO 4 nanorods before and after net-
working with MWCNT. With both the pristine and MWCNT networked samples,
the shorter LiFePO 4 nanorods exhibit higher discharge capacity at a given C-rate
than the long nanorods due to a faster lithium-ion diffusion arising from a shorter
diffusion length. With both the long and short nanorods, the LiFePO 4 /MWCNT