Page 391 - Handbook of Battery Materials
P. 391
362 12 Lithium Intercalation Cathode Materials for Lithium-Ion Batteries
form of LiCoO 2 , which has a lithiated spinel structure [30]. The extraction of lithium
from the 8a sites occurs at around 3.9 V, and the insertion of additional lithium
into 16c sites occurs at around 3.5 V. However, the system suffers from a huge po-
larization loss, as indicated by a large separation between the charge and discharge
profiles. Attempts to make LiNi 2 O 4 spinel by chemically extracting 50% of lithium
◦
from LiNiO 2 followed by heating at 200 C results in a spinel-like cubic phase, but
◦
with the Ni 3+/4+ ions occupying both the 16c and 16d sites. Heating at T > 200 C
results in a disproportionation of cubic LiNi 2 O 4 into LiNiO 2 and NiO [92].
12.14
Polyanion-containing Cathodes
Although simple oxides such as LiCoO 2 , LiNiO 2 , and LiMn 2 O 4 with highly oxidized
redox couples (Co 3+/4+ ,Ni 3+/4+ ,Mn 3+/4+ , respectively) were able to offer high cell
voltages of ∼4 V in lithium-ion cells, they are prone to release oxygen from the lattice
in the charged state at elevated temperatures because of the chemical instability of
4+
highly oxidized species such as Co 4+ and Ni . One way to overcome this problem
is to work with lower-valent redox couples like Fe 2+/3+ . However, a decrease in the
oxidation state raises the redox energy of the cathode and lowers the cell voltage.
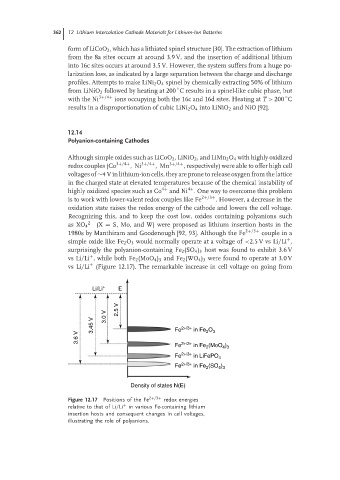
Recognizing this, and to keep the cost low, oxides containing polyanions such
2− (X = S, Mo, and W) were proposed as lithium insertion hosts in the
as XO 4
1980s by Manthiram and Goodenough [92, 93]. Although the Fe 2+/3+ couple in a
+
simple oxide like Fe 2 O 3 would normally operate at a voltage of <2.5 V vs Li/Li ,
surprisingly the polyanion-containing Fe 2 (SO 4 ) 3 host was found to exhibit 3.6 V
+
vs Li/Li , while both Fe 2 (MoO 4 ) 3 and Fe 2 (WO 4 ) 3 were found to operate at 3.0 V
+
vs Li/Li (Figure 12.17). The remarkable increase in cell voltage on going from
Li/Li + E
2.5 V
3.0 V
3.45 V Fe 2+/3+ in Fe O 3
2
3.6 V
Fe 2+/3+ in Fe (MoO )
4 3
2
2+/3+
Fe in LiFePO 4
2+/3+
Fe in Fe (SO )
2
4 3
Density of states N(E)
Figure 12.17 Positions of the Fe 2+/3+ redox energies
relative to that of Li/Li + in various Fe-containing lithium
insertion hosts and consequent changes in cell voltages,
illustrating the role of polyanions.