Page 387 - Handbook of Battery Materials
P. 387
358 12 Lithium Intercalation Cathode Materials for Lithium-Ion Batteries
120 25 °C
110
100
90
Capacity (mAh/g) 120 60 °C
80
70
100
80
60
0 10 20 30 40 50
Cycle number
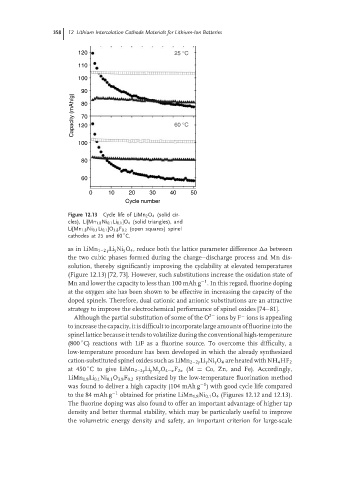
Figure 12.13 Cycle life of LiMn 2 O 4 (solid cir-
cles), Li[Mn 1.8 Ni 0.1 Li 0.1 ]O 4 (solid triangles), and
Li[Mn 1.8 Ni 0.1 Li 0.1 ]O 3.8 F 0.2 (open squares) spinel
◦
cathodes at 25 and 60 C.
as in LiMn 1–2 y Li y Ni y O 4 , reduce both the lattice parameter difference a between
the two cubic phases formed during the charge–discharge process and Mn dis-
solution, thereby significantly improving the cyclability at elevated temperatures
(Figure 12.13) [72, 73]. However, such substitutions increase the oxidation state of
−1
Mn and lower the capacity to less than 100 mAh g . In this regard, fluorine doping
at the oxygen site has been shown to be effective in increasing the capacity of the
doped spinels. Therefore, dual cationic and anionic substitutions are an attractive
strategy to improve the electrochemical performance of spinel oxides [74–81].
−
Although the partial substitution of some of the O 2− ions by F ions is appealing
to increase the capacity,it is difficult to incorporate large amounts of fluorine into the
spinel lattice because it tends to volatilize during the conventional high-temperature
◦
(800 C) reactions with LiF as a fluorine source. To overcome this difficulty, a
low-temperature procedure has been developed in which the already synthesized
cation-substituted spinel oxides such as LiMn 2–2y Li y Ni y O 4 are heated with NH 4 HF 2
◦
at 450 C to give LiMn 2–2y Li y M y O 4−x F 2x (M = Co, Zn, and Fe). Accordingly,
LiMn 1.8 Li 0.1 Ni 0.1 O 3.8 F 0.2 synthesized by the low-temperature fluorination method
−1
was found to deliver a high capacity (104 mAh g ) with good cycle life compared
to the 84 mAh g −1 obtained for pristine LiMn 1.8 Ni 0.1 O 4 (Figures 12.12 and 12.13).
The fluorine doping was also found to offer an important advantage of higher tap
density and better thermal stability, which may be particularly useful to improve
the volumetric energy density and safety, an important criterion for large-scale