Page 382 - Handbook of Battery Materials
P. 382
12.8 Li[Li 1/3 Mn 2/3 ]O 2 -LiMO 2 Solid Solutions 353
5 300 x = 0.3
4 x = 0.3 250
3
2 200
5 x = 0.4
4 x = 0.4 250
3 200
Voltage (V) 2 x = 0.5 Discharge capacity (mAh/g) 300 x = 0.5
5
300
4
250
3
2
5 200 x = 0.6
300
4
x = 0.6 250
3
2 Unmodified 200
5 Al 2 O 3 modified 300 Unmodified
4 250 Al 2 O 3 modified x = 0.7
3 x = 0.7
2 200
0 50 100 150 200 250 300 350 0 5 10 15 20 25 30
(a) Specific capacity (mAh/g) (b) Cycle number
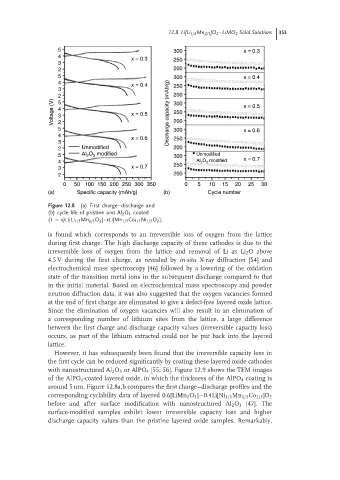
Figure 12.8 (a) First charge–discharge and
(b) cycle life of pristine and Al 2 O 3 coated
(1 – x)Li[Li 1/3 Mn 2/3 O 2 ]- xLi[Mn 1/3 Co 1/3 Ni 1/3 O 2 ].
is found which corresponds to an irreversible loss of oxygen from the lattice
during first charge. The high discharge capacity of these cathodes is due to the
irreversible loss of oxygen from the lattice and removal of Li as Li 2 O above
4.5 V during the first charge, as revealed by in-situ X-ray diffraction [54] and
electrochemical mass spectroscopy [46] followed by a lowering of the oxidation
state of the transition metal ions in the subsequent discharge compared to that
in the initial material. Based on electrochemical mass spectroscopy and powder
neutron diffraction data, it was also suggested that the oxygen vacancies formed
at the end of first charge are eliminated to give a defect-free layered oxide lattice.
Since the elimination of oxygen vacancies will also result in an elimination of
a corresponding number of lithium sites from the lattice, a large difference
between the first charge and discharge capacity values (irreversible capacity loss)
occurs, as part of the lithium extracted could not be put back into the layered
lattice.
However, it has subsequently been found that the irreversible capacity loss in
the first cycle can be reduced significantly by coating these layered oxide cathodes
with nanostructured Al 2 O 3 or AlPO 4 [55, 56]. Figure 12.9 shows the TEM images
of the AlPO 4 -coated layered oxide, in which the thickness of the AlPO 4 coating is
around 5 nm. Figure 12.8a,b compares the first charge–discharge profiles and the
corresponding cyclability data of layered 0.6[LiMn 2 O 3 ]−0.4Li[Ni 1/3 Mn 1/3 Co 1/3 ]O 2
before and after surface modification with nanostructured Al 2 O 3 [47]. The
surface-modified samples exhibit lower irreversible capacity loss and higher
discharge capacity values than the pristine layered oxide samples. Remarkably,