Page 379 - Handbook of Battery Materials
P. 379
350 12 Lithium Intercalation Cathode Materials for Lithium-Ion Batteries
250
Specific capacity (mAh/g) 200 umodified LiCoO 2 (4.3 - 3.2 V)
150
100
umodified LiCoO 2 (4.5 - 3.2 V)
2
Al 2 O 3 -modified LiCoO 2 (4.3 - 3.2 V)
50 umodified LiCoO (4.7 - 3.2 V)
Al 2 O 3 -modified LiCoO 2 (4.5 - 3.2 V)
Al O 3 -modified LiCoO 2 (4.7 - 3.2 V)
2
0
0 20 40 60 80 100
Cycle number
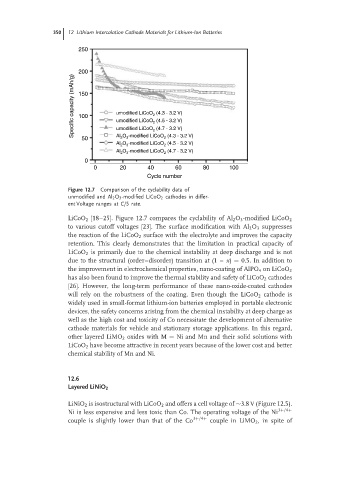
Figure 12.7 Comparison of the cyclability data of
unmodified and Al 2 O 3 -modified LiCoO 2 cathodes in differ-
ent Voltage ranges at C/5 rate.
LiCoO 2 [18–25]. Figure 12.7 compares the cyclability of Al 2 O 3 -modified LiCoO 2
to various cutoff voltages [23]. The surface modification with Al 2 O 3 suppresses
the reaction of the LiCoO 2 surface with the electrolyte and improves the capacity
retention. This clearly demonstrates that the limitation in practical capacity of
LiCoO 2 is primarily due to the chemical instability at deep discharge and is not
due to the structural (order–disorder) transition at (1 – x) = 0.5. In addition to
the improvement in electrochemical properties, nano-coating of AlPO 4 on LiCoO 2
has also been found to improve the thermal stability and safety of LiCoO 2 cathodes
[26]. However, the long-term performance of these nano-oxide-coated cathodes
will rely on the robustness of the coating. Even though the LiCoO 2 cathode is
widely used in small-format lithium-ion batteries employed in portable electronic
devices, the safety concerns arising from the chemical instability at deep charge as
well as the high cost and toxicity of Co necessitate the development of alternative
cathode materials for vehicle and stationary storage applications. In this regard,
other layered LiMO 2 oxides with M = Ni and Mn and their solid solutions with
LiCoO 2 have become attractive in recent years because of the lower cost and better
chemical stability of Mn and Ni.
12.6
Layered LiNiO 2
LiNiO 2 is isostructural with LiCoO 2 and offers a cell voltage of ∼3.8 V (Figure 12.5).
Ni is less expensive and less toxic than Co. The operating voltage of the Ni 3+/4+
couple is slightly lower than that of the Co 3+/4+ couple in LiMO 2 ,inspite of