Page 378 - Handbook of Battery Materials
P. 378
12.5 Layered LiCoO 2 349
Table 12.1 Crystal field stabilization energies (CFSEs) and
octahedral site stabilization energies (OSSE) of some 3d
transition metal ions.
d
Ion Octahedral coordination Tetrahedral coordination OSSE (Dq)
b
Configuration a CFSE (Dq) Configuration a CFSE b,c (Dq)
2
2 0
3+
V :3d 2 t e 0 −8 e t 2 −5.33 −2.67
2g g
3
2 1
Cr :3d 3 t e 0 −12 e t (HS) −3.56 −8.44
3+
2
2g g
3
2 2
1
Mn :3d 4 t e (HS) −6 e t (HS) −1.78 −4.22
3+
2g g
2
2
3
2 3
3+
Fe :3d 5 t e (HS) 0 e t (HS) 0 0
2
2g g
0
6
3 3
3+
Co :3d 6 t e (LS) −24 e t (HS) −2.67 −21.33
2g g 2
6
4 3
1
3+
Ni :3d 7 t e (LS) −18 e t (HS) −5.33 −12.67
2g g
2
a LS and HS refer, respectively, to low-spin and high-spin configurations.
b
Pairing energies are neglected for simplicity.
c Obtained by assuming t = 0.444 o ; t and o refer, respectively, to tetrahedral and octahedral
splittings.
d Obtained by taking the difference between the CFSE values for octahedral and tetrahedral
coordinations.
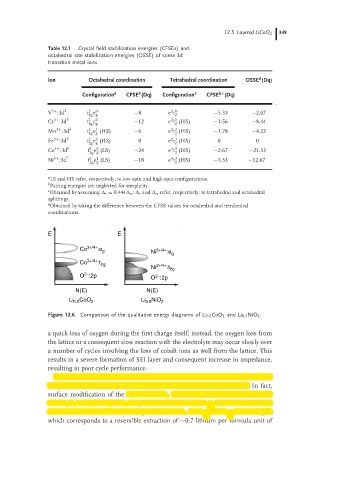
E E
Co 3+/4+ :e g 3+/4+
Ni :e g
Co 3+/4+ :t 2g
Ni 3+/4+ :t 2g
2−
O :2p O :2p
2−
N(E) N(E)
CoO Li NiO
Li 0.5 2 0.5 2
Figure 12.6 Comparison of the qualitative energy diagrams of Li 0.5 CoO 2 and Li 0.5 NiO 2 .
a quick loss of oxygen during the first charge itself; instead, the oxygen loss from
the lattice or a consequent slow reaction with the electrolyte may occur slowly over
a number of cycles involving the loss of cobalt ions as well from the lattice. This
results in a severe formation of SEI layer and consequent increase in impedance,
resulting in poor cycle performance.
One way to suppress the chemical instability or reactivity with the electrolyte
is to coat or modify the surface of the cathode with other inert oxides. In fact,
surface modification of the layered LiCoO 2 cathode with nanostructured oxides
like Al 2 O 3 ,TiO 2 ,ZrO 2 , SiO 2 ,MgO,ZnO,and MPO 4 (M = Al and Fe) has been
−1
found to increase the reversible capacity of LiCoO 2 from ∼140 to ∼ 200 mAh g ,
which corresponds to a reversible extraction of ∼0.7 lithium per formula unit of