Page 376 - Handbook of Battery Materials
P. 376
12.4 Layered Metal Oxide Cathodes 347
12.4
Layered Metal Oxide Cathodes
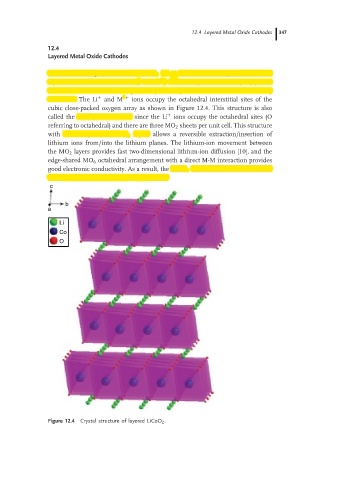
Oxides with the general formula LiMO 2 (M = V, Cr, Co, and Ni) crystallize in a
+
layered structure in which the Li and M 3+ ions occupy the alternate (111) planes
of the rock salt structure to give a layered sequence of –O–Li–O–M–O– along
the c axis. The Li + and M 3+ ions occupy the octahedral interstitial sites of the
cubic close-packed oxygen array as shown in Figure 12.4. This structure is also
+
called the O3 layered structure, since the Li ions occupy the octahedral sites (O
referring to octahedral) and there are three MO 2 sheets per unit cell. This structure
with covalently bonded MO 2 layers allows a reversible extraction/insertion of
lithium ions from/into the lithium planes. The lithium-ion movement between
the MO 2 layers provides fast two-dimensional lithium-ion diffusion [10], and the
edge-shared MO 6 octahedral arrangement with a direct M-M interaction provides
good electronic conductivity. As a result, the LiMO 2 oxides have become attractive
cathode candidates for lithium-ion batteries.
c
b
a
Li
Co
O
Figure 12.4 Crystal structure of layered LiCoO 2 .