Page 377 - Handbook of Battery Materials
P. 377
348 12 Lithium Intercalation Cathode Materials for Lithium-Ion Batteries
12.5
Layered LiCoO 2
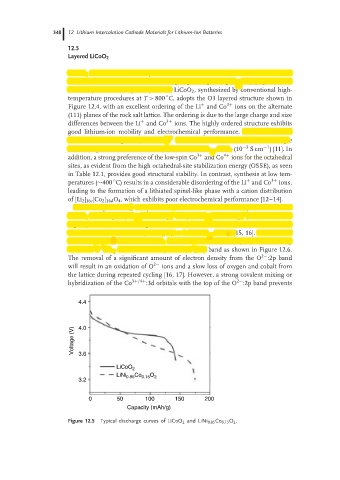
LiCoO 2 is the most commonly used transition metal oxide cathode in commercial
lithium-ion batteries because of its high operating voltage (∼4 V) (Figure 12.5),
ease of synthesis, and good cycle life. LiCoO 2 , synthesized by conventional high-
◦
temperature procedures at T > 800 C, adopts the O3 layered structure shown in
+
Figure 12.4, with an excellent ordering of the Li and Co 3+ ions on the alternate
(111) planes of the rock salt lattice. The ordering is due to the large charge and size
+
differences between the Li and Co 3+ ions. The highly ordered structure exhibits
good lithium-ion mobility and electrochemical performance. The direct Co-Co
interaction with a partially filled t 6−x band associated with the Co 3+/4+ couple
2g
−1
leads to high electronic conductivity (metallic) for Li 1−x CoO 2 (10 −3 Scm ) [11]. In
addition, a strong preference of the low-spin Co 3+ and Co 4+ ions for the octahedral
sites, as evident from the high octahedral-site stabilization energy (OSSE), as seen
in Table 12.1, provides good structural stability. In contrast, synthesis at low tem-
+
◦
peratures (∼400 C) results in a considerable disordering of the Li and Co 3+ ions,
leading to the formation of a lithiated spinel-like phase with a cation distribution
of [Li 2 ] 16c [Co 2 ] 16d O 4 , which exhibits poor electrochemical performance [12–14].
+
Even though one Li ion per formula unit can be theoretically extracted from
−1
−1
LiCoO 2 with a capacity of ∼274 mAh g , only 50% (∼140 mAh g ) of its theoretical
capacity can be utilized in practical lithium-ion cells because of structural and
chemical instabilities at deep charge (x > 0.5inLi 1−x CoO 2 ) [15, 16]. Extraction of
+
more than 0.5 Li ions from LiCoO 2 leads to chemical instability due to the overlap
2−
of the Co 3+/4+ :t 2g band with the top of the O :2p band as shown in Figure 12.6.
2−
The removal of a significant amount of electron density from the O :2p band
will result in an oxidation of O 2− ions and a slow loss of oxygen and cobalt from
the lattice during repeated cycling [16, 17]. However, a strong covalent mixing or
2−
hybridization of the Co 3+/4+ :3d orbitals with the top of the O :2p band prevents
4.4
Voltage (V) 4.0
3.6
LiCoO 2
LiNi 0.85 Co 0.15 O 2
3.2
0 50 100 150 200
Capacity (mAh/g)
Figure 12.5 Typical discharge curves of LiCoO 2 and LiNi 0.85 Co 0.15 O 2 .