Page 375 - Handbook of Battery Materials
P. 375
346 12 Lithium Intercalation Cathode Materials for Lithium-Ion Batteries
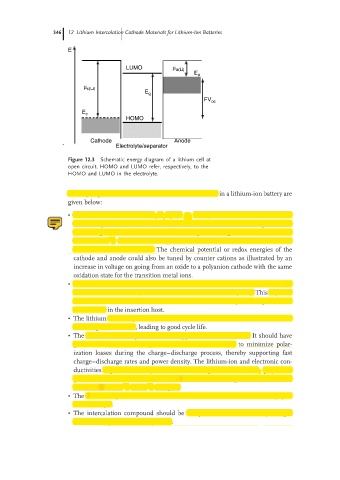
E
LUMO µ a(Li)
E a
µ c(Li)
E g
FV oc
E c
HOMO
Cathode Anode
Electrolyte/separator
Figure 12.3 Schematic energy diagram of a lithium cell at
open circuit. HOMO and LUMO refer, respectively, to the
HOMO and LUMO in the electrolyte.
The key requirements for a successful cathode material in a lithium-ion battery are
given below:
• The intercalation cathode Li x M y X z (X = anion) should have a low lithium
chemical potential, and the intercalation anode should have a high lithium
chemical potential to maximize the cell voltage. This implies that the transition
metal ion M n+ should have a high oxidation state in the cathode and a low
oxidation state in the anode. The chemical potential or redox energies of the
cathode and anode could also be tuned by counter cations as illustrated by an
increase in voltage on going from an oxide to a polyanion cathode with the same
oxidation state for the transition metal ions.
• The intercalation compound should allow for insertion/extraction of a large
number of lithium ions per formula unit to maximize cell capacity. This depends
on the number of available lithium sites and the accessibility of multiple valence
states for M in the insertion host.
• The lithium insertion/extraction reaction should be reversible, with minimal or
no change in structure, leading to good cycle life.
• The intercalation compound should support mixed conduction. It should have
good electronic conductivity and lithium-ion conductivity to minimize polar-
ization losses during the charge–discharge process, thereby supporting fast
charge–discharge rates and power density. The lithium-ion and electronic con-
ductivities depend on the crystal structure, arrangement of the MX n polyhedral
geometry, interconnection of lithium sites, electronic configuration, and relative
positions of the M n+ and X n− energies.
• The redox energies of the cathode and anode should lie within the band gap of
the electrolyte.
• The intercalation compound should be inexpensive, environmentally benign,
and thermally and chemically stable.