Page 386 - Handbook of Battery Materials
P. 386
12.11 Spinel LiMn 2 O 4 357
4.4
LiMn 2 O 4
4.0
3.6
4C 2C 1C C/2 C/5 C/10
4.4 LiMn 1.8 Li 0.2 O 4
4.0 C/5
C/10
3.6 4C 2C 1C C/2
Voltage vs. Li/Li + (V) 4.0 4C 2C 1C O 3.79 0.21 C/5
4.4
Li
F
LiMn
0.2
1.8
C/10
3.6
C/2
4.4
LiMn 1.8 Li 0.1 Ni 0.1 O 4
4.0 C/5
3.6 4C 2C 1C C/2 C/10
4.4
LiMn 1.8 Li 0.1 Ni 0.1 O 3.8 F 0.2
4.0 C/5
3.6 4C 2C 1C C/2 C/10
0 20 40 60 80 100 120
Capacity (mAh/g)
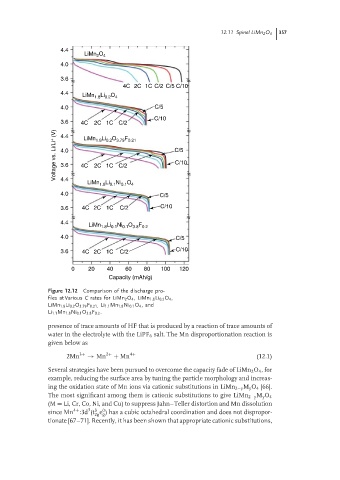
Figure 12.12 Comparison of the discharge pro-
files at Various C rates for LiMn 2 O 4 ,LiMn 1.8 Li 0.2 O 4 ,
LiMn 1.8 Li 0.2 O 3.79 F 0.21, Li 1.1 Mn 1.8 Ni 0.1 O 4 ,and
Li 1.1 Mn 1.8 Ni 0.1 O 3.8 F 0.2 .
presence of trace amounts of HF that is produced by a reaction of trace amounts of
water in the electrolyte with the LiPF 6 salt. The Mn disproportionation reaction is
given below as
2Mn 3+ → Mn 2+ + Mn 4+ (12.1)
Several strategies have been pursued to overcome the capacity fade of LiMn 2 O 4 , for
example, reducing the surface area by tuning the particle morphology and increas-
ing the oxidation state of Mn ions via cationic substitutions in LiMn 2−y M y O 4 [66].
The most significant among them is cationic substitutions to give LiMn 2−y M y O 4
(M = Li, Cr, Co, Ni, and Cu) to suppress Jahn–Teller distortion and Mn dissolution
3 3
0
since Mn :3d (t e ) has a cubic octahedral coordination and does not dispropor-
4+
2g g
tionate [67–71]. Recently, it has been shown that appropriate cationic substitutions,