Page 389 - Handbook of Battery Materials
P. 389
360 12 Lithium Intercalation Cathode Materials for Lithium-Ion Batteries
spinel encounters the formation of NiO impurity during synthesis, and the
ordering between Mn 4+ and Ni 2+ leads to inferior performance compared to the
disordered phase [87]. It has been found that the formation of the NiO impurity
phase and ordering can be suppressed by appropriate cationic substitutions, as in
LiMn 1.5 Ni 0.42 Zn 0.08 O 4 and LiMn 1.42 Ni 0.42 Co 0.16 O 4 [88].
One major concern with the spinel LiMn 1.5 Ni 0.5 O 4 cathode is the chemical
stability in contact with the electrolyte at the higher operating voltage of 4.7 V. To
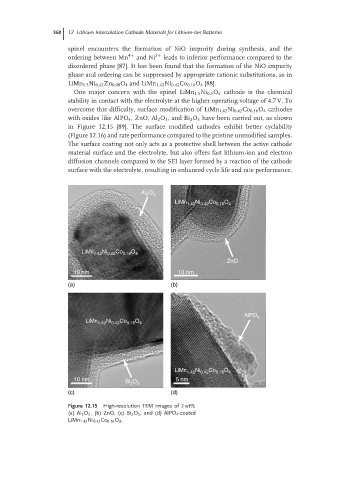
overcome this difficulty, surface modification of LiMn 1.42 Ni 0.42 Co 0.16 O 4 cathodes
with oxides like AlPO 4 ,ZnO, Al 2 O 3 ,and Bi 2 O 3 have been carried out, as shown
in Figure 12.15 [89]. The surface modified cathodes exhibit better cyclability
(Figure 12.16) and rate performance compared to the pristine unmodified samples.
The surface coating not only acts as a protective shell between the active cathode
material surface and the electrolyte, but also offers fast lithium-ion and electron
diffusion channels compared to the SEI layer formed by a reaction of the cathode
surface with the electrolyte, resulting in enhanced cycle life and rate performance.
Al O 3
2
LiMn 1.42 Ni 0.42 Co 0.16 O 4
Ni Co O
LiMn 1.42 0.42 0.16 4
ZnO
10 nm 10 nm
(a) (b)
AlPO 4
LiMn 1.42 Ni 0.42 Co 0.16 O 4
LiMn 1.42 Ni 0.42 Co 0.16 O 4
10 nm 5 nm
Bi 2 O 3
(c) (d)
Figure 12.15 High-resolution TEM images of 2 wt%
(a) Al 2 O 3 , (b) ZnO, (c) Bi 2 O 3 ,and (d) AlPO 4 -coated
LiMn 1.42 Ni 0.42 Co 0.16 O 4 .