Page 388 - Handbook of Battery Materials
P. 388
12.12 5 V Spinel Oxides 359
electric vehicle applications. Another strategy that has been pursued to improve the
cyclability of the LiMn 2 O 4 spinel is surface modification or coating of its surface
with other oxides such as LiCoO 2 ,ZrO 2 , SiO 2 ,V 2 O 5 ,Al 2 O 3 , or MgO with the aim
of minimizing the contact of the cathode surface with the electrolyte and thereby
suppressing the dissolution of manganese. In fact, the surface oxides coated on
LiMn 2 O 4 have been found to suppress Mn dissolution from the spinel lattice in
contact with the electrolyte and improve the capacity retention [82–84].
12.12
5 V Spinel Oxides
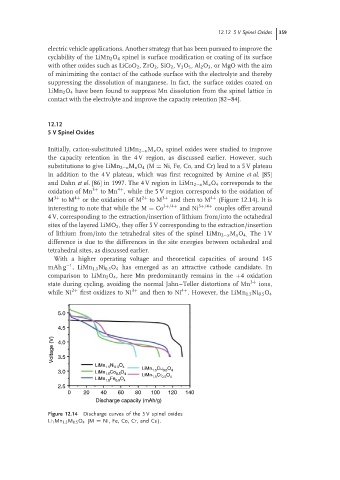
Initially, cation-substituted LiMn 2−x M x O 4 spinel oxides were studied to improve
the capacity retention in the 4 V region, as discussed earlier. However, such
substitutions to give LiMn 2−x M x O 4 (M = Ni, Fe, Co, and Cr) lead to a 5 V plateau
in addition to the 4 V plateau, which was first recognized by Amine et al. [85]
and Dahn et al. [86] in 1997. The 4 V region in LiMn 2−x M x O 4 corresponds to the
4+
oxidation of Mn 3+ to Mn , while the 5 V region corresponds to the oxidation of
M 3+ to M 4+ or the oxidation of M 2+ to M 3+ and then to M 4+ (Figure 12.14). It is
interesting to note that while the M = Co 3+/4+ and Ni 3+/4+ couples offer around
4 V, corresponding to the extraction/insertion of lithium from/into the octahedral
sites of the layered LiMO 2 , they offer 5 V corresponding to the extraction/insertion
of lithium from/into the tetrahedral sites of the spinel LiMn 2−x M x O 4. The 1 V
difference is due to the differences in the site energies between octahedral and
tetrahedral sites, as discussed earlier.
With a higher operating voltage and theoretical capacities of around 145
−1
mAh g ,LiMn 1.5 Ni 0.5 O 4 has emerged as an attractive cathode candidate. In
comparison to LiMn 2 O 4 , here Mn predominantly remains in the +4oxidation
state during cycling, avoiding the normal Jahn–Teller distortions of Mn 3+ ions,
while Ni 2+ first oxidizes to Ni 3+ and then to Ni . However, the LiMn 1.5 Ni 0.5 O 4
4+
5.0
4.5
Voltage (V) 4.0
3.5
LiMn 1.5 Ni 0.5 O 4
3.0 LiMn 1.5 Co 0.5 O 4 LiMn 1.5 Cu 0.5 O 4
LiMn 1.5 Cr 0.5 O 4
LiMn 1.5 Fe 0.5 O 4
2.5
0 20 40 60 80 100 120 140
Discharge capacity (mAh/g)
Figure 12.14 Discharge curves of the 5 V spinel oxides
Li 1 Mn 1.5 M 0.5 O 4 (M = Ni, Fe, Co, Cr, and Cu).