Page 396 - Handbook of Battery Materials
P. 396
12.15 Phospho-Olivine LiMPO 4 367
nanocomposites exhibit higher capacity at a given C-rate than the pristine LiFePO 4
due to the enhancement in electronic conductivity.
Although the initial work by Goodenough’s group revealed a two-phase reaction
mechanism with LiFePO 4 and FePO 4 as end members, subsequent investigations
have indicated several interesting observations [112–115]. For example, the mis-
cibility gap between the two phases has been found to decrease with increasing
temperature [113], and the occurrence of a single-phase solid solution Li x FePO 4
◦
with 0 ≤ x ≤ 1 has been reported at 450 C. Similarly, the miscibility gap has
been found to decrease with decreasing particle size, and complete solid solubility
between LiFePO 4 and FePO 4 at room temperature has been reported for 40 nm
size particles [114, 115]. Thus, what was originally found to be a two-phase reaction
mechanism with micrometer-sized particles has now turned into a single-phase
reaction mechanism with nano-sized particles. This is a clear demonstration of
how nanoparticles can behave entirely differently from their micrometer-sized
counterparts. Defects caused by the existence of cationic vacancies in the samples
prepared at low temperatures have been suggested to contribute to the unique
behavior of the nano-sized particles.
2+
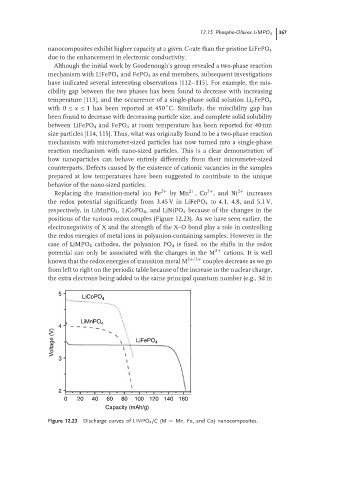
Replacing the transition-metal ion Fe 2+ by Mn ,Co , and Ni 2+ increases
2+
the redox potential significantly from 3.45 V in LiFePO 4 to 4.1, 4.8, and 5.1 V,
respectively, in LiMnPO 4 ,LiCoPO 4 , and LiNiPO 4 because of the changes in the
positions of the various redox couples (Figure 12.23). As we have seen earlier, the
electronegativity of X and the strength of the X–O bond play a role in controlling
the redox energies of metal ions in polyanion-containing samples. However in the
case of LiMPO 4 cathodes, the polyanion PO 4 is fixed, so the shifts in the redox
potential can only be associated with the changes in the M 2+ cations. It is well
known that the redox energies of transition metal M 2+/3+ couples decrease as we go
from left to right on the periodic table because of the increase in the nuclear charge,
the extra electrons being added to the same principal quantum number (e.g., 3d in
5
LiCoPO 4
LiMnPO 4
4
Voltage (V) LiFePO 4
3
2
0 20 40 60 80 100 120 140 160
Capacity (mAh/g)
Figure 12.23 Discharge curves of LiMPO 4 /C (M = Mn, Fe, and Co) nanocomposites.