Page 414 - Handbook of Battery Materials
P. 414
386 13 Rechargeable Lithium Anodes
13.7.1
Electrolytes
Lithium cycling efficiency depends on the electrolyte solutions used. A mixture
of EC and 2MeTHF with LiAsF 6 as the solute exhibits a high lithium cycling
efficiency of 97.2% (FOM = 35.7) as revealed by an Li-on-Li half-cell test with
◦
10 −2 Scm −1 conductivity at 25 C [47, 48]. A Li/a-V 2 O 5 –P 2 O 5 coin-type cell with
LiAsF 6 –EC/2MeTHF has an FOM of 28.2, while cells with 2MeTHF or EC/PC (1 : 1)
have FOMs of 9.4 and 14.8, respectively [48]. (a-V 2 O 5 means amorphous V 2 O 5 .)
Lithium cycling efficiency is strongly influenced by impurities in electrolytes. The
relationship between total impurity and the FOM of Li in LiAsF 6 –EC/2MeTHF
has been examined [49]. The FOM for EC/2MeTHF increases with decreases in
both water and organic impurities. The influence of the impurity depends on the
electrolyte system used.
Hayashi et al. [50] investigated the electrolyte materials and their compositions
with various carbonates and ethers as solvents in relation to the cycling efficiency of
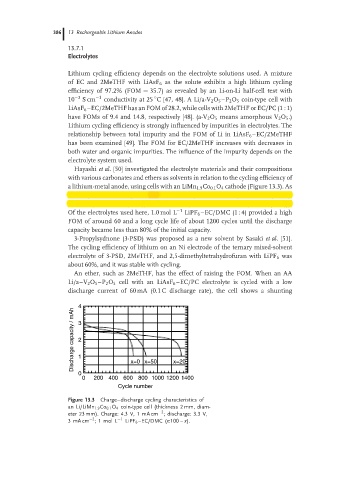
a lithium-metal anode, using cells with an LiMn 1.9 Co 0.1 O 4 cathode (Figure 13.3). As
electrolyte solvents, they used four carbonates (PC, EC, dimethyl carbonate (DMC),
and diethyl carbonate (DEC)) and two ethers (DME and 1, 2-diethoxyethane (DEE))).
Of the electrolytes used here, 1.0 mol L −1 LiPF 6 –EC/DMC (1 : 4) provided a high
FOM of around 60 and a long cycle life of about 1200 cycles until the discharge
capacity became less than 80% of the initial capacity.
3-Propylsydnone (3-PSD) was proposed as a new solvent by Sasaki et al. [51].
The cycling efficiency of lithium on an Ni electrode of the ternary mixed-solvent
electrolyte of 3-PSD, 2MeTHF, and 2,5-dimethyltetrahydrofuran with LiPF 6 was
about 60%, and it was stable with cycling.
An ether, such as 2MeTHF, has the effect of raising the FOM. When an AA
Li/a–V 2 O 5 –P 2 O 5 cell with an LiAsF 6 –EC/PC electrolyte is cycled with a low
discharge current of 60 mA (0.1 C discharge rate), the cell shows a shunting
4
Discharge capacity / mAh 3
2
1
0 x=0 x=50 x=20
0 200 400 600 800 1000 1200 1400
Cycle number
Figure 13.3 Charge–discharge cycling characteristics of
an Li/LiMn 1.9 Co 0.1 O 4 coin-type cell (thickness 2 mm, diam-
−2
eter 23 mm). Charge: 4.3 V, 1 mA cm ; discharge: 3.3 V,
−2
3mA cm ;1 molL −1 LiPF 6 –EC/DMC (x:100 – x).