Page 417 - Handbook of Battery Materials
P. 417
13.7 Improvement in the Cycling Efficiency of a Lithium Anode 389
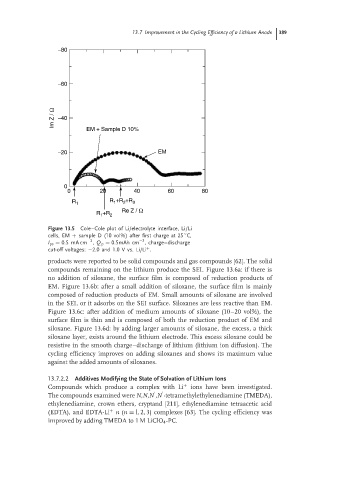
−80
−60
Im Z / Ω −40
EM + Sample D 10%
−20 EM
0
0 20 40 60 80
R +R +R
R 1 1 2 3
Re Z / Ω
R 1 +R 2
Figure 13.5 Cole–Cole plot of Li/electrolyte interface, Li/Li
cells, EM + sample D (10 vol%) after first charge at 25 C,
◦
−2
−2
I ps = 0.5mA cm , Q p = 0.5mAh cm , charge–discharge
+
cut-off voltages: −2.0 and 1.0 V vs. Li/Li .
products were reported to be solid compounds and gas compounds [62]. The solid
compounds remaining on the lithium produce the SEI. Figure 13.6a: if there is
no addition of siloxane, the surface film is composed of reduction products of
EM. Figure 13.6b: after a small addition of siloxane, the surface film is mainly
composed of reduction products of EM. Small amounts of siloxane are involved
in the SEI, or it adsorbs on the SEI surface. Siloxanes are less reactive than EM.
Figure 13.6c: after addition of medium amounts of siloxane (10–20 vol%), the
surface film is thin and is composed of both the reduction product of EM and
siloxane. Figure 13.6d: by adding larger amounts of siloxane, the excess, a thick
siloxane layer, exists around the lithium electrode. This excess siloxane could be
resistive in the smooth charge–discharge of lithium (lithium ion diffusion). The
cycling efficiency improves on adding siloxanes and shows its maximum value
against the added amounts of siloxanes.
13.7.2.2 Additives Modifying the State of Solvation of Lithium Ions
Compounds which produce a complex with Li + ions have been investigated.
The compounds examined were N,N,N ,N -tetramethylethylenediamine (TMEDA),
ethylenediamine, crown ethers, cryptand [211], ethylenediamine tetraacetic acid
(EDTA), and EDTA-Li n (n = l, 2, 3) complexes [63]. The cycling efficiency was
+
improved by adding TMEDA to 1 M LiClO 4 -PC.