Page 422 - Handbook of Battery Materials
P. 422
394 13 Rechargeable Lithium Anodes
The lithium deposition morphology is compact and smooth in PC with added
polysulfide, whereas it is dendritic in PC alone.
Matsuda and co-workers [80, 81] examined LiI, SnI 2 ,AlI 3 , and 2MeF as addi-
tives in LiClO 4 –PC or LiClO 4 –PC/DMC electrolyte. They measured the cycling
efficiency of lithium on an Ni electrode. All the additives increased the efficiency,
the best additive being a combination of AlI 3 and 2MeF. They attributed the
improvement to the formation of Li–Al alloy on the surface by AlI 3 or to a more
protective film formed by 2MeF.
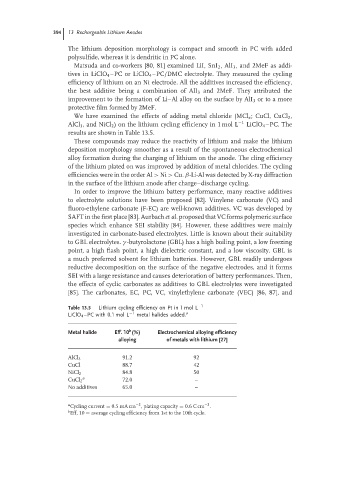
We have examined the effects of adding metal chloride (MCl x ; CuCl, CuCl 2 ,
AlCl 3 ,and NiCl 2 ) on the lithium cycling efficiency in 1 mol L −1 LiClO 4 –PC. The
results are shown in Table 13.5.
These compounds may reduce the reactivity of lithium and make the lithium
deposition morphology smoother as a result of the spontaneous electrochemical
alloy formation during the charging of lithium on the anode. The cling efficiency
of the lithium plated on was improved by addition of metal chlorides. The cycling
efficiencies were in the order Al > Ni > Cu. β-Li-Al was detected by X-ray diffraction
in the surface of the lithium anode after charge–discharge cycling.
In order to improve the lithium battery performance, many reactive additives
to electrolyte solutions have been proposed [82]. Vinylene carbonate (VC) and
fluoro-ethylene carbonate (F-EC) are well-known additives. VC was developed by
SAFT in the first place [83]. Aurbach et al. proposed that VC forms polymeric surface
species which enhance SEI stability [84]. However, these additives were mainly
investigated in carbonate-based electrolytes. Little is known about their suitability
to GBL electrolytes. γ -butyrolactone (GBL) has a high boiling point, a low freezing
point, a high flash point, a high dielectric constant, and a low viscosity. GBL is
a much preferred solvent for lithium batteries. However, GBL readily undergoes
reductive decomposition on the surface of the negative electrodes, and it forms
SEI with a large resistance and causes deterioration of battery performances. Then,
the effects of cyclic carbonates as additives to GBL electrolytes were investigated
[85]. The carbonates, EC, PC, VC, vinylethylene carbonate (VEC) [86, 87], and
Table 13.5 Lithium cycling efficiency on Pt in 1 mol L −1
LiClO 4 –PC with 0.1 mol L −1 metal halides added. a
b
Metal halide Eff. 10 (%) Electrochemical alloying efficiency
alloying of metals with lithium [27]
AlCl 3 91.2 92
CuCl 88.7 42
NiCl 2 84.8 50
a
CuCl 2 72.0 –
No additives 65.0 –
a Cycling current = 0.5mA cm , plating capacity = 0.6C cm .
−2
−2
b
Eff, 10 = average cycling efficiency from 1st to the 10th cycle.