Page 420 - Handbook of Battery Materials
P. 420
392 13 Rechargeable Lithium Anodes
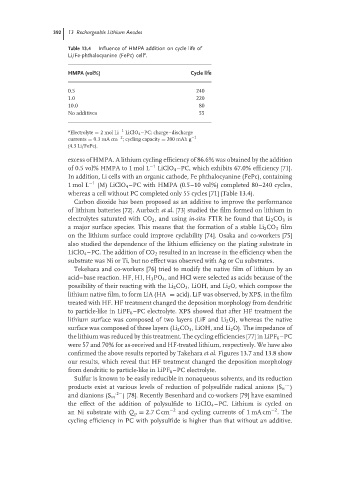
Table 13.4 Influence of HMPA addition on cycle life of
a
Li/Fe-phthalocyanine (FePc) cell .
HMPA (vol%) Cycle life
0.5 240
1.0 220
10.0 80
No additives 55
a −1
Electrolyte = 2molLi LiClO 4 –PC; charge–discharge
−2
currents = 0.3mA cm ; cycling capacity = 200 mAh g −1
(4.3 Li/FePc).
excess of HMPA. A lithium cycling efficiency of 86.6% was obtained by the addition
of 0.5 vol% HMPA to 1 mol L −1 LiClO 4 –PC, which exhibits 67.0% efficiency [71].
In addition, Li cells with an organic cathode, Fe phthalocyanine (FePc), containing
1 mol L −1 (M) LiClO 4 –PC with HMPA (0.5–10 vol%) completed 80–240 cycles,
whereas a cell without PC completed only 55 cycles [71] (Table 13.4).
Carbon dioxide has been proposed as an additive to improve the performance
of lithium batteries [72]. Aurbach et al. [73] studied the film formed on lithium in
electrolytes saturated with CO 2 , and using in-situ FTIR he found that Li 2 CO 3 is
a major surface species. This means that the formation of a stable Li 2 CO 3 film
on the lithium surface could improve cyclability [74]. Osaka and co-workers [75]
also studied the dependence of the lithium efficiency on the plating substrate in
LiClO 4 –PC. The addition of CO 2 resulted in an increase in the efficiency when the
substrate was Ni or Ti, but no effect was observed with Ag or Cu substrates.
Tekehara and co-workers [76] tried to modify the native film of lithium by an
acid–base reaction. HF, HI, H 3 PO 4 , and HCl were selected as acids because of the
possibility of their reacting with the Li 2 CO 3 ,LiOH, andLi 2 O, which compose the
lithium native film, to form LiA (HA = acid). LiF was observed, by XPS, in the film
treated with HF. HF treatment changed the deposition morphology from dendritic
to particle-like in LiPF 6 –PC electrolyte. XPS showed that after HF treatment the
lithium surface was composed of two layers (LiF and Li 2 O), whereas the native
surface was composed of three layers (Li 2 CO 3 , LiOH, and Li 2 O). The impedance of
the lithium was reduced by this treatment. The cycling efficiencies [77] in LiPF 6 –PC
were 57 and 70% for as-received and HF-treated lithium, respectively. We have also
confirmed the above results reported by Takehara et al. Figures 13.7 and 13.8 show
our results, which reveal that HF treatment changed the deposition morphology
from dendritic to particle-like in LiPF 6 –PC electrolyte.
Sulfur is known to be easily reducible in nonaqueous solvents, and its reduction
products exist at various levels of reduction of polysulfide radical anions (S n )
·−
·2−
and dianions (S m ) [78]. Recently Besenhard and co-workers [79] have examined
the effect of the addition of polysulfide to LiClO 4 –PC. Lithium is cycled on
−2
an Ni substrate with Q p = 2.7C cm −2 and cycling currents of 1 mA cm . The
cycling efficiency in PC with polysulfide is higher than that without an additive.