Page 418 - Handbook of Battery Materials
P. 418
390 13 Rechargeable Lithium Anodes
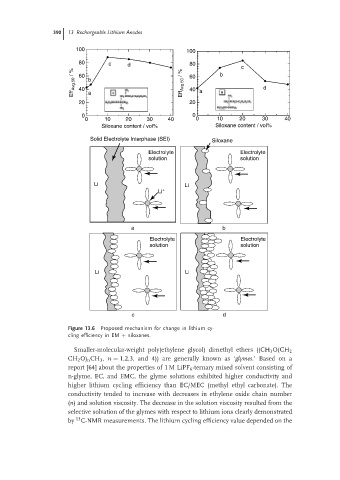
100 100
80 c d 80 c
Eff Avg.50 / % 60 b Eff Avg.50 / % 60 a b d
40
40
20 a 20
0 0
0 10 20 30 40 0 10 20 30 40
Siloxane content / vol% Siloxane content / vol%
Solid Electrolyte Interphase (SEI) Siloxane
Electrolyte Electrolyte
solution solution
Li Li
Li +
a b
Electrolyte Electrolyte
solution solution
Li Li
c d
Figure 13.6 Proposed mechanism for change in lithium cy-
cling efficiency in EM + siloxanes.
Smaller-molecular-weight poly(ethylene glycol) dimethyl ethers ((CH 3 O(CH 2
CH 2 O) n CH 3 , n = 1,2,3, and 4)) are generally known as ‘glymes.’ Based on a
report [64] about the properties of 1 M LiPF 6 -ternary mixed solvent consisting of
n-glyme, EC, and EMC, the glyme solutions exhibited higher conductivity and
higher lithium cycling efficiency than EC/MEC (methyl ethyl carbonate). The
conductivity tended to increase with decreases in ethylene oxide chain number
(n) and solution viscosity. The decrease in the solution viscosity resulted from the
selective solvation of the glymes with respect to lithium ions clearly demonstrated
by 13 C-NMR measurements. The lithium cycling efficiency value depended on the