Page 56 - Handbook of Battery Materials
P. 56
22 1 Thermodynamics and Mechanistics
1,6
1,4
U t.v. [V] 1,2
1,0
0,8
0,6
0,0 0,5 1,0 1,5 2,0
I [A]
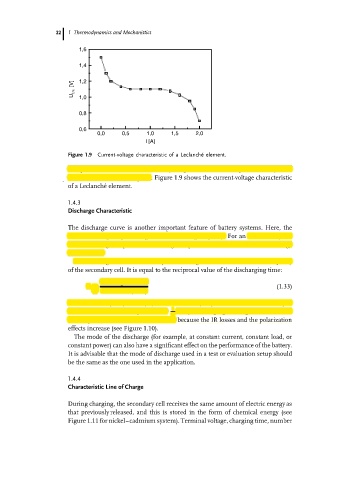
Figure 1.9 Current-voltage characteristic of a Leclanch´ e element.
The power as a function of the battery weight is known as the power density P s of
the element in watts/kilogram. Figure 1.9 shows the current-voltage characteristic
of a Leclanch´ eelement.
1.4.3
Discharge Characteristic
The discharge curve is another important feature of battery systems. Here, the
terminal voltage is plotted against the discharge capacity. For an ideal battery the
terminal voltage drops to zero in a single step when the whole of the stored energy
is consumed.
The discharge rate C is defined by the discharge current and the nominal capacity
of the secondary cell. It is equal to the reciprocal value of the discharging time:
discharge current
C = (1.33)
nominal capacity
The nominal capacity of every system is defined by a specific value of C; for example,
for th nickel–cadmium system, it is 1 C. By discharging at a higher current, the
20
final capacity obtainable becomes lower because the IR losses and the polarization
effects increase (see Figure 1.10).
The mode of the discharge (for example, at constant current, constant load, or
constant power) can also have a significant effect on the performance of the battery.
It is advisable that the mode of discharge used in a test or evaluation setup should
be the same as the one used in the application.
1.4.4
Characteristic Line of Charge
During charging, the secondary cell receives the same amount of electric energy as
that previously released, and this is stored in the form of chemical energy (see
Figure 1.11 for nickel–cadmium system). Terminal voltage, charging time, number