Page 624 - Handbook of Battery Materials
P. 624
598 17 Liquid Nonaqueous Electrolytes
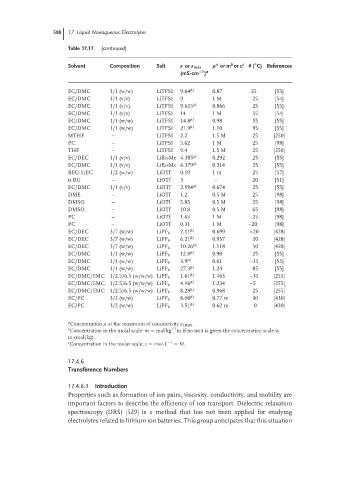
Table 17.17 (continued)
b
a
◦
Solvent Composition Salt κ or κ max µ or m or c c θ ( C) References
−1 a
(mS·cm )
EC/DMC 1/1 (w/w) LiTFSI 9.64 µ) 0.87 25 [55]
EC/DMC 1/1 (v/v) LiTFSI 9 1 M 25 [54]
EC/DMC 1/1 (v/v) LiTFSI 9.615 µ) 0.866 25 [55]
EC/DMC 1/1 (v/v) LiTFSI 14 1 M 55 [54]
EC/DMC 1/1 (w/w) LiTFSI 14.8 µ) 0.98 55 [55]
EC/DMC 1/1 (w/w) LiTFSI 21.9 µ) 1.10 95 [55]
MTHF – LiTFSI 2.2 1.5 M 25 [250]
PC – LiTFSI 5.62 1 M 25 [98]
THF – LiTFSI 9.4 1.5 M 25 [250]
EC/DEC 1/1 (v/v) LiBisMe 4.385 µ) 0.292 25 [55]
EC/DMC 1/1 (v/v) LiBisMe 6.379 µ) 0.314 25 [55]
BEG-1/EC 1/2 (w/w) LiOTf 0.19 1 m 25 [57]
n-BU – LiOTf 3 – 20 [51]
EC/DMC 1/1 (v/v) LiOTf 2.994 µ) 0.674 25 [55]
DME – LiOTf 1.2 0.5 M 25 [98]
DMSO – LiOTf 5.85 0.5 M 25 [98]
DMSO – LiOTf 10.8 0.5 M 65 [98]
PC – LiOTf 1.65 1 M 25 [98]
PC – LiOTf 0.31 1 M –20 [98]
EC/DEC 3/7 (w/w) LiPF 6 2.11 µ) 0.699 –20 [428]
EC/DEC 3/7 (w/w) LiPF 6 6.21 µ) 0.957 20 [428]
EC/DEC 3/7 (w/w) LiPF 6 10.26 µ) 1.118 50 [428]
EC/DMC 1/1 (w/w) LiPF 6 12.0 µ) 0.90 25 [55]
EC/DMC 1/1 (w/w) LiPF 6 3.9 µ) 0.61 –15 [55]
EC/DMC 1/1 (w/w) LiPF 6 27.3 µ) 1.24 85 [55]
EC/DMC/EMC 1/2.5/6.5 (w/w/w) LiPF 6 1.61 µ) 1.463 –35 [255]
EC/DMC/EMC 1/2.5/6.5 (w/w/w) LiPF 6 4.46 µ) 1.234 –5 [255]
EC/DMC/EMC 1/2.5/6.5 (w/w/w) LiPF 6 8.28 µ) 0.968 25 [255]
EC/PC 3/2 (w/w) LiPF 6 8.00 µ) 0.77 m 30 [430]
EC/PC 3/2 (w/w) LiPF 6 3.51 µ) 0.62 m 0 [430]
a
Concentration µ at the maximum of conductivity κ max .
b Concentration in the molal scale: m = mol·kg −1 m if no unit is given the concentration scale is
m·(mol/kg).
c Concentration in the molar scale: c = mol·L −1 = M.
17.4.6
Transference Numbers
17.4.6.1 Introduction
Properties such as formation of ion pairs, viscosity, conductivity, and mobility are
important factors to describe the efficiency of ion transport. Dielectric relaxation
spectroscopy (DRS) [529] is a method that has not been applied for studying
electrolytes related to lithium ion batteries. This group anticipates that this situation