Page 619 - Handbook of Battery Materials
P. 619
17.4 Bulk Properties 593
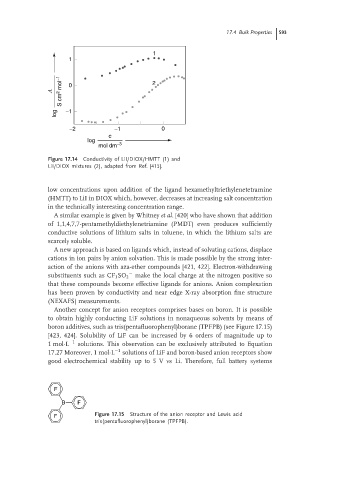
1
1
S cm 2 mol −1
L 0 2
log −1
−2 −1 0
c
log
mol dm −3
Figure 17.14 Conductivity of LiI/DIOX/HMTT (1) and
LiI/DIOX mixtures (2), adapted from Ref. [415].
low concentrations upon addition of the ligand hexamethyltriethylenetetramine
(HMTT) to LiI in DIOX which, however, decreases at increasing salt concentration
in the technically interesting concentration range.
A similar example is given by Whitney et al. [420] who have shown that addition
of 1,1,4,7,7-pentamethyldiethylenetriamine (PMDT) even produces sufficiently
conductive solutions of lithium salts in toluene, in which the lithium salts are
scarcely soluble.
A new approach is based on ligands which, instead of solvating cations, displace
cations in ion pairs by anion solvation. This is made possible by the strong inter-
action of the anions with aza-ether compounds [421, 422]. Electron-withdrawing
−
substituents such as CF 3 SO 2 make the local charge at the nitrogen positive so
that these compounds become effective ligands for anions. Anion complexation
has been proven by conductivity and near edge X-ray absorption fine structure
(NEXAFS) measurements.
Another concept for anion receptors comprises bases on boron. It is possible
to obtain highly conducting LiF solutions in nonaqueous solvents by means of
boron additives, such as tris(pentafluorophenyl)borane (TPFPB) (see Figure 17.15)
[423, 424]. Solubility of LiF can be increased by 6 orders of magnitude up to
1 mol·L −1 solutions. This observation can be exclusively attributed to Equation
17.27 Moreover, 1 mol·L −1 solutions of LiF and boron-based anion receptors show
good electrochemical stability up to 5 V vs Li. Therefore, full battery systems
F
B F
F Figure 17.15 Structure of the anion receptor and Lewis acid
tris(pentafluorophenyl)borane (TPFPB).